Bioresorbable vascular scaffold (BVS) devices have represented an authentic conceptual revolution in interventional cardiology.1–6 Their particular design ensures perfect scaffolding for the vascular wall and has led to excellent immediate outcomes. Furthermore, they incorporate a drug with potent antiproliferative properties, which averts the development of restenosis.2–6 These 2 properties are also inherent to drug-eluting stents (DES) made of metal. Nonetheless, the attractiveness of BVS is that once their function has been achieved (vascular support and antiproliferative effect), both the scaffold and the polymer used to administer the drug completely disappear from the coronary wall.2–6 In contrast, with DES a metallic structure always remains in the vascular wall, and in those not containing a bioresorbable polymer, the permanent polymer covering the stent also persists.7,8 Several studies have conclusively confirmed that BVS completely disappear from the vascular wall over time, usually within a period of around 3 years.2,3 This implies that the artery will be released from the corset-like effect of a metallic mesh structure in its interior and can recover its physiologic functions.7,8 The vessel can respond once again to the stimuli generated by the coronary flow (sheer stress), which may favor chronic phenomena of adaptive vascular remodeling and late lumen gain. Recovery of the physiologic vascular dynamics is also achieved, with restoration of acute vasodilation or vasoconstriction responses to various stimuli and drugs.2,3 Some data have even indicated that regression of the underlying atheromatous plaque can occur in the treated region,2,3 and that implantation of BVS over vulnerable or complicated plaques may help to stabilize them.
Resorption also frees lateral branch vessels that have been “caged” by the scaffold. Moreover, the eventual disappearance of BVS structural elements that were improperly placed against the vessel wall (malapposition) due to an inadequate technique or unfavorable anatomy, and those that protrude excessively (in ostial lesions) may avoid the development of late complications.2–6 Finally, the nonmetallic structure of these scaffolds (with a platinum marker at each end) enables proper evaluation of the coronary anatomy by noninvasive techniques (eg, coronary computed tomography) because it does not produce radiologic artifacts, as occurs with metallic stents.2–6
There is some evidence that the permanent presence of foreign elements in the vessel wall may promote the development of adverse events during follow-up. Very late thrombosis causes the greatest concern, but late restenosis has also been described, sometimes caused by neoatherosclerosis.7,8 BVS were designed in an attempt to circumvent all these limitations, associated with DES.
Numerous studies have reported excellent clinical results following BVS implantation.2–5 Observational studies and randomized studies performed in selected patients have both reported outcomes similar to those achieved with latest-generation DES.2–5 If the 1-year results obtained with these scaffolds are similar to those of the newest DES, it is tempting to speculate that the very long-term outcome may also be favorable for BVS-treated patients. We should remember, however, that the currently available scaffolds contain relatively thick support elements (156 μm) to ensure sufficient radial strength; therefore, they are inferior to the new generations of DES in terms of flexibility and navigability. This explains why their use has been constrained and cautious in patients with complex or calcified lesions. Furthermore, shaping and adaptation of current BVS to the vessel is very limited because of their plastic composition. Therefore, the diameters of these devices must be carefully chosen, as excessive expansion (or dilatation of the cells in the case of lateral branches) can cause fracture and disruption of the support elements.2–6 These problems rarely occur with DES, which allow for greater adaptation while maintaining their structural integrity within the limits required in clinical practice. These factors explain why the favorable initial results obtained with BVS (similar to those of the newest DES) are applicable to relatively straightforward lesions.2–5
As has always occurred in the history of interventional cardiology, every innovation is understandably accompanied by an initial phase of enthusiasm, which at some point becomes subdued by data that generate concern and reflection within the scientific community.9 One only has to recall the provocative editorial published not long ago in this same journal, predicting that we had achieved every interventional cardiologist's dream: a 0% restenosis rate!10 However, reality soon returned us to a more cautious and humble scenario.1,7 Usually, the next phase of an innovation entails incorporation of additional technological advances, and the new devices are better used. The initial limitations are overcome and the innovation becomes consolidated, which facilitates its generalized use.7 Recently, the enthusiasm generated by the development of BVS and their excellent preliminary results have been eclipsed by the emergence of some potential drawbacks.9,11,12 Meta-analyses of the related studies have detected a clear indication that BVS-treated patients have an increased risk of thrombosis.11,12 Similarly, these studies show that the late angiographic outcomes are slightly inferior to those obtained with latest-generation DES.12 In this line, the results at 3 years of follow-up reported in the recently published ABSORB II study were not only unable to confirm recovery of the vascular dynamics in the BVS-treated segment, but also point to poorer late angiographic results and a higher rate of adverse clinical events (revascularization requirement and device thrombosis) than with the new DES.6 Hence, we are now experiencing a new phase of concern and reflection regarding the usefulness of BVS in clinical practice.
DIAGNOSTIC TECHNIQUES USED WITH BIORESORBABLE VASCULAR SCAFFOLDSIntravascular diagnostic techniques can help to optimize BVS implantation and study the changes undergone by the scaffolds over time. Optical coherence tomography (OCT) has a resolution of 15 μm, 10-fold greater than that achieved by intravascular ultrasound. It provides extremely high-quality tomographic images of the coronary walls and the results following implantation of intravascular devices.13 Because of this unprecedented resolution, the technique enables precise analysis of the residual lumen, the degree of expansion of the BVS, apposition of its structural elements to the coronary wall, prolapse of material (atheroma or thrombus) into the vessel, and the development of dissections at the stent edges. The sensitivity of OCT for the diagnosis of all these phenomena is much higher than that of intravascular ultrasound, although the clinical significance of the more minor changes is uncertain.13 During follow-up, OCT can be used to visualize the degree of coating of the scaffold's structural elements and the proliferative response in the interior, and eventually, to confirm its degradation and ultimate disappearance from the vessel.3 Nonetheless, perhaps one of the most interesting contributions of this technique is its ability to characterize the tissue created within the BVS, and specifically, to detect the presence of neoatherosclerosis or plaque rupture.8,13–18
The use of intravascular ultrasound also enables detection of mechanical problems derived from suboptimal BVS implantation, and because of its greater tissue penetration, provides a more complete spatial vision than OCT of both the underlying atheroma plaque and expansion of the device with respect to the total area of the vessel (external elastic lamina). Virtual histology, which is also based on ultrasound, is useful for detecting changes in the BVS composition as it undergoes resorption.3
Lastly, because of their nonmetallic characteristics, BVS can also be examined noninvasively. In this line, coronary computed tomography can be of particular value in the follow-up of selected patients treated with these scaffolds (proximal vessels with a suitable size) for both clinical and research purposes.2,3
CLINICAL PROBLEMS ASSOCIATED WITH BIORESORBABLE VASCULAR SCAFFOLDSAt this time, there is little information regarding the pathophysiologic mechanisms implicated in the specific complications associated with BVS. The available reports only include single cases or short, retrospective series of patients who experienced BVS thrombosis or restenosis,15–18 and it has been found that these 2 events are closely related in some patients. There is also evidence that many of the pathophysiologic mechanisms implicated in thrombosis or restenosis of conventional bare metal stents or DES can also affect BVS.8,19 However, some specific characteristics of BVS, such as the thickness of their structural elements, their plastic properties, and the actual process of resorption, may also explain some of the late failures of these devices.
The various mechanisms implicated in BVS restenosis are summarized in Table15–18 (Figure). They are diverse, and several mechanisms can coincide in the same patient. Inadequate expansion of the scaffold, as well as fracture due to overexpansion, can both be associated with prompt or late failure. Small size of the target vessel is a classic risk factor for the development of stenosis, regardless of the type of coronary procedure carried out, although the greater thickness of current BVS may explain their reduced effectiveness in small vessels. Some BVS restenoses have occurred in long lesions requiring overlapping of various scaffolds. Even in perfectly expanded BVS, abundant neointimal proliferation can cause restenosis. It is unknown whether this is attributable to resistance to the drug used. Neoatherosclerosis seems to be a particularly relevant problem in all antiproliferative drug-eluting devices.20 In contrast to the neointimal hyperplasia caused by relatively homogeneous muscle cell proliferation (classic substrate of stent restenosis), neoatherosclerosis implies the formation of true fibroatheroma plaques within the stent. Although these plaques usually contain a high lipid content, some can progress to calcified plaques. Neoatherosclerosis is common and can occur promptly following DES implantation.20 Recently, this factor was described as a cause of BVS restenosis. The most characteristic presentation is thin-cap fibroatheroma. It has been suggested that complicated neoatherosclerosis (thin-cap fibroatheroma rupture with associated thrombosis) may be the link between restenosis and stent thrombosis, until recently considered to be completely independent entities.8 This phenomenon has also been detected in analyses of the causes of late BVS failure.15–17 The problem of unsuccessful coverage of the entire segment to be treated with the device (“geographical miss”), described in relation to DES, has also been found to affect BVS. Furthermore, the strategy of performing more aggressive predilatation with systematic postdilatation of BVS can explain the development of small dissections, which are almost universally detected at the scaffold edges using OCT.
Mechanisms Implicated in Restenosis of Bioresorbable Vascular Scaffold Systems
| 1. | Excessive neointimal proliferation | |
| 2. | Neoatherosclerosis | Stable (gradual development) Complicated (capsule rupture with associated thrombosis) |
| 3. | Underexpansion of the scaffold with preserved structure | |
| 4. | Target vessel too small (< 2 mm) (strut overcrowding) | |
| 5. | Scaffold structural changes | Acute: • Due to damage during implantation or inappropriate overexpansion (BVS fracture) • Due to insufficient relative radial strength: acute collapse (acute recoil) |
| Late: due to programmed resorption • Within the wall (due to a loss of structural support) – Maintaining their configuration and position – With displacement, disruption or late collapse (late recoil). Loss of alignment or circularity. • With disrupted elements within the lumen (outside the wall) (poor apposition) – Maintaining their configuration – Changing their spatial configuration. Loss of alignment or circularity | ||
| 6. | “Delayed” scaffold resorption. Very late persistence (> 3-4 y) of BVS structural elements | |
| 7. | Resistance to antiproliferative drug | |
| 8. | Adjacent segment not covered by the scaffold | • Disease progression (plaques having a different composition) at the initially untreated edges (5 mm adjacent to the BVS) • Progression of the adjacent atherosclerotic plaque, which was treated, but went uncovered by the scaffold (geographical miss) • Overlapping failure (gap) between 2 scaffolds |
| 9. | Excessive overlapping of adjacent scaffolds (long lesions) |
BVS, bioresorbable vascular scaffold.
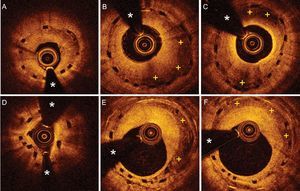
Optical coherence tomography (OCT) images in patients with restenosis of bioresorbable vascular scaffold (BVS) devices. A: Substantial underexpansion of a BVS. The neointimal growth has a bright and relatively homogeneous appearance. B: Severe neointimal proliferation, but showing clear areas of attenuation (+) in a properly expanded BVS. C: Proliferation having a heterogeneous appearance, with very bright intima near the lumen and broad areas of attenuation that partially obscure the structural elements of a morphologically elliptical BVS. D: BVS disruption, seen as an absence of continuity and circularity of the structural elements, with moderate associated neointimal growth. E and F: Restenosis of a BVS caused by heterogeneous tissue, with areas of attenuation (+) showing well-delimited borders (E). The BVS had been implanted to treat restenosis of a metallic stent. The structural elements of the BVS are visualized as “black boxes” with no shadowing, whereas the struts of the metallic stent are seen as very bright focal areas with posterior shadowing. *: guidewire artifact.
There are 2 problems inherent to BVS use that may have implications in the development of restenosis. First, their lower radial strength and potential for disruption during an aggressive implantation can explain some early recurrences.2–6 Second, gradual loss of the capacity for support derived from programmed resorption may favor the development of late restenosis due to progressive elastic recoil of the vessel wall.2–6 Although gradual resorption of the BVS does not imply a change in its configuration under normal conditions, it has been hypothesized that in some patients it may be associated with considerable spatial changes, loss of alignment of the structural elements, and overlapping of the struts.16–18 The clinical implications of these phenomena would likely be limited when they occur within the vessel wall. However, disruption of the BVS elements outside the wall (within the vessel lumen) has been associated with restenosis problems such as very late thrombosis.18 Unexpectedly, in some patients with very late BVS failure, persistent structural material has been detected, when it should have completely disappeared according to the time elapsed since implantation.18 New studies are undoubtedly needed to gain information on the phenomena associated with BVS resorption and their clinical implications, especially when these scaffolds are used in anatomically unfavorable situations.
CURRENT STUDYThe study by Chavarría el al.21 published in Revista Española de Cardiología, analyzes the clinical, angiographic, and OCT characteristics of 17 patients with BVS restenosis. The results are of considerable interest because of the meticulous analyses carried out and the scant information available on this uncommon complication. The series was derived from a total population of 330 patients who underwent BVS implantation (398 BVS to treat 380 lesions), rigorously followed up for 19 ± 10 months. The use of coronary computed tomography angiography in all patients during follow-up, and OCT analysis in all those who developed stenosis, lends particular value to the study. Eighteen BVS with restenosis were detected in 17 patients, yielding a restenosis incidence of 5.4%. Computed tomography showed low-density, noncalcified tissue as the cause of the new lesion. The mean time to the development of restenosis was 9±4 months. The most common morphology was a focal pattern (12 patients, 67%) that usually affected the proximal edge of the scaffold (9 patients, 75%). Among the 9 patients with compromise of the edge, 3 also showed a lesion within the BVS, and in the remaining 6, the lesion was located immediately outside the scaffold. When these focal restenoses affected the interior of the scaffold, the tissue had a heterogeneous or layered appearance. However, in 6 patients (33%), restenosis showed a diffuse morphology. In these cases, OCT depicted a lipid pattern or layered pattern, associated with microcalcifications and microvessels, all features indicative of neoatherosclerosis. In total, almost half the lesions showed features consistent with neoatherosclerosis. Of interest, available OCT images taken immediately after scaffold implantation in 10 patients enabled comparison with those obtained at the time of restenosis. In one-third of these patients, postprocedure OCT showed significant underexpansion of the BVS, which may have favored later development of restenosis. Furthermore, serial studies showed that late lumen loss was never a consequence of collapse of the BVS structure or elastic recoil. In addition, it is relevant that in patients with focal restenosis at the scaffold edge, postprocedure OCT showed significant lipid plaques in that position. Also of note, in 5 of the 18 BVS restenoses, certain elements were seen to be completely overlapped, indicating that major disruption of the scaffold had occurred in the late phase of degradation. In fact, in 3 cases of BVS disruption during follow-up, immediate postprocedure OCT showed correctly expanded struts without overlapping. Finally, the authors found that early restenoses (<6 months) tended to be more focal, affected the BVS edges, and showed tissue with a homogeneous appearance. In contrast, late stenoses had a more diffuse angiographic pattern and showed heterogeneous tissue, often with clear features of neoatherosclerosis.21
Retreatment involved DES implantation in all patients, except in 4 with restenosis at the proximal edge just outside the scaffold, who underwent implantation of another BVS.
In this study, restenoses were documented at a time when the BVS structure was still in existence. New studies are needed to characterize the very late restenosis patterns, once the scaffold has completely disappeared.
CONCLUSIONSAt a time when there is ongoing controversy about the indications for BVS implantation in daily practice2–12,22 and the usefulness of bioresorbable scaffolds in relatively adverse clinical and anatomical situations, studies such as that by Chavarría et al.21 are of enormous interest. Investigation should continue to better characterize the mechanisms implicated in late and very late BVS failures (restenosis and thrombosis). In this line, the prospective RIBS VII study, focused on evaluating treatment for BVS restenosis in Spain, may provide particularly relevant data. In addition, the clinical and anatomic scenarios in which scaffolds can provide better outcomes and clear benefits to patients should be identified. It seems reasonable that optimized implantation of BVS can improve their safety and effectiveness, and the systematic use of intravascular imaging techniques can aid in this regard.13 However, we should humbly recognize that we were fully aware of these technical and methodological considerations when we began using these devices some years ago. Finally, the currently available data indicate that the appropriate duration and type of antiplatelet therapy used following BVS implantation should be reconsidered.6–11,15–18 New generations of BVS (with significant improvements in the classic polylactic acid scaffold or with biocorrosive magnesium scaffolds) will soon be available for generalized clinical use, and are expected to overcome many of the limitations of the current scaffolds. Nonetheless, rigorous critical evaluations of the results obtained with the new scaffolds should always follow the dictum that governs the development of interventional cardiology: Treatment for our patients should not and cannot be based on simple expectations, no matter how attractive they may be.22
CONFLICTS OF INTERESTNone declared.