The prevalence of resistant hypertension has recently been reported, but there are no studies on its demography. This study aimed to examine the demography and clinical characteristics of resistant hypertension in a large sample of primary care patients.
MethodsA cross-sectional study was performed of all computerized medical records of hypertensive patients in Health Area 6 of Madrid (Spain). Of 63 167 hypertensive patients, we selected 48 744 with prescription of antihypertensive medication; of these, we selected those who met the American Heart Association criteria for resistant hypertension.
ResultsA total of 6292 patients had resistant hypertension, representing 9.9% of all hypertensive patients and 12.9% of those treated. A total of 5.5% were < 50 years (8.5% men and 3.2% women) and 24.7% were > 80 years (15.8% men and 31.4% women) (P < .001). In patients < 50 years, resistant hypertension was associated with male sex (odds ratio female/male = 0.006; 95% confidence interval, 0.000-0.042; P < .001), systolic blood pressure, obesity, stroke, and chronic kidney disease (P < .001). In those > 80 years, resistant hypertension was associated with female sex (odds ratio female/male = 1.27; 95% confidence interval, 1.08-1,10; P = .004), systolic blood pressure, diabetes mellitus, obesity, chronic kidney disease, coronary heart disease, and atrial fibrillation (P < .001). More than 50% of patients > 80 years with resistant hypertension had cardiovascular disease.
ConclusionsOne in 4 patients with resistant hypertension is > 80 years. Resistant hypertension is associated with cardiovascular disease, age < 50 years in men and age > 80 years in women. There is a high proportion of cardiovascular disease in elderly patients with resistant hypertension.
Keywords
Resistant hypertension, defined as failure to control blood pressure (BP) >140/90 mmHg despite the concomitant use of 3 or more antihypertensive medications, including 1 diuretic at optimal or best tolerated dose, or with BP controlled despite the use of 4 or more medications, seems to be an important problem in clinical practice.1,2 Since the publication of the American Heart Association BP guidelines on resistant hypertension in 2008,1 very few studies have reported data on the prevalence of resistant hypertension and associated conditions.3–7 This contrasts with the figures reported in clinical trials, which range over a wide interval between 15% and 30%.8–10 A few population-based studies such as the Framingham Heart Study,11 the National Health and Nutrition Examination Survey, and the Spanish Ambulatory Blood Pressure Monitoring Registry have identified age, race, diabetes mellitus (DM), associated cardiovascular disease (CVD), and chronic kidney disease as predictors of resistant hypertension.4,12 However, there is a relative lack of data on hypertensive patients < 50 years and > 80 years,13,14 and we have found no studies on the demography of resistant hypertension. We consider that such studies could be important because of progressive aging in developed countries and because they could also provide additional, potentially relevant information, both from the epidemiologic and clinical practice points of view. It is estimated that 19.3% of Americans will be > 65 years by 2030 and in 2050 the number of Americans aged 65 years and older is projected to be 88.5 million, more than double its projected population of 40.2 million in 2010.15 Most hypertension guidelines provide no recommendations specific to the elderly population, except the American College of Cardiology Foundation/American Heart Association consensus statement16–20 and the very recently reported 2013 European Society of Hypertension/European Society of Cardiology guidelines.21
This study had 2 objectives: a) to estimate the frequency and clinical characteristics of resistant hypertension in a large sample of all hypertensive patients managed in a primary care setting, and b) to analyze the demography of resistant hypertension.
METHODSDesignWe designed a cross-sectional study based on computerized registries of hypertensive men and women belonging to Health Area 6 of Madrid. Patients were included if they had attended their health center in 2008 for monitoring and to request prescriptions; this definition represents almost all hypertensive patients on drug treatment, whether or not they are treated in primary care, since most people go to their health center for prescriptions. Of the whole population, 63 167 patients met the criterion of adequate data quality to carry out the analysis. From this group, we first selected the 48 746 persons who had received prescriptions for antihypertensive drugs and, of these; we selected those who met the criteria for resistant hypertension. In accordance with the American Heart Association definition, patients were considered to have resistant hypertension if their systolic blood pressure (SBP) was ≥ 140 and/or and diastolic BP ≥ 90mmHg while taking 3 antihypertensive drugs, including 1 diuretic, or if they were taking 4 or more drugs, regardless of whether they were controlled.1 Hypertension was considered to exist if it had been previously diagnosed or if the clinical history documented 3 BP measurements in the consultation ≥ 140/90mmHg on 3 different days in a 3-month period, or 1 measurement of ≥ 180/110mmHg, under usual conditions of clinical practice with predominantly aneroid sphygmomanometers calibrated annually according to a standard protocol. BP was considered to be controlled if the last 2 measurements on 2 different dates were < 140/90mmHg, in accordance with the recommendations of the European guidelines.16–21
Variables StudiedThe variables selected were age, sex, smoking habits, SBP and diastolic BP (mmHg), weight (kg), height (cm), body mass index (kg/m2), total cholesterol (mg/dL), low density lipoprotein cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), and creatinine (mg/dL). The estimated glomerular filtration rate (mL/min/1.73 m2) was calculated using the Modification of Diet in Renal Disease-4 formula.22 The laboratory variables were taken from samples obtained primarily in the health centers in baseline conditions after an 8-h fast and were sent to the 2 reference laboratories for the area. The morbidity analyzed was the presence of a previous diagnosis in the clinical history according to ICPC-2 23 codes for the following conditions: DM (T90), which considers the diagnostic criteria of random glucose test ≥ 11mmol/L or 200mg/dL with classic symptoms of DM, 2 or more random glucose tests ≥ 7 mmol/L or 126mg/dL and 2 or more glucose tests ≥ 11 mmol/L or 200mg/dL 2h after oral loading with 75g of glucose; hyperlipidemia (T93) using the following criteria: 2 measurements of total cholesterol ≥ 6.57 mmol/L (250mg/dL) or ≥ 5.18 mmol/L (200mg/dL) if there is DM or CVD; obesity (T82), if body mass index ≥ 30kg/m2; and smoking (P17) for consumption of any amount of tobacco.
The following diagnoses (usually taken from hospital discharge reports) were considered in the clinical history: coronary heart disease (K74, K76), heart failure (K77), peripheral arterial disease (K99), chronic kidney disease (U99), stroke (K89, K90), and atrial fibrillation (K78). CVD was considered if some of the conditions were present.
Antihypertensive drug prescriptions were analyzed according to the classification by therapeutic groups of the Anatomical Therapeutic Chemical Classification System, which is the European system for coding drugs and medications.24 The following drug classes were analyzed: antihypertensive (C02); diuretics (C03); aldosterone antagonists alone or in combination (C03D,C03E); alpha-blockers (C02C); beta blockers (C07); calcium channel blockers (C08); and inhibitors of the renin-angiotensin-aldosterone system, either angiotensin converting enzyme inhibitors (C09) or angiotensin receptor blockers, alone or in combination.
Data AnalysisThe data was reviewed and checked for possible coding errors, and frequency distributions were calculated. The Kolmogorov-Smirnov test was used to test the normality of the variables. We used basic central tendency statistics: the arithmetic mean (standard deviation) for continuous variables, and relative distribution of frequencies (prevalence) for categorical variables, with their 95% confidence intervals 95%CI. For comparison of means between groups we used Student's t test for binary independent variables, and for comparisons of proportions we used Pearson's Chi-square test. Logistic regression analysis was performed to identify the variables independently associated with resistant hypertension. We selected the sociodemographic and clinical variables that were statistically significant in the bivariate analyses. Multiple logistic regression analysis was performed by sequentially entering into the model all independent variables with a P value < .05, that is, age, sex,smoking, SBP, diastolic BP, glomerular filtration rate, hyperlipidemia, obesity, DM, peripheral arterial disease, chronic kidney disease, coronary heart disease, and stroke. Stepwise logistic regression was investigated separately in the total population and by age (< 50 years, 50-79 years, > 80 years) because an interaction was found between sex, age and resistant hypertension. The variables that remained in the final model were considered when they all reached statistical significance as independent predictors. Data are presented as odds ratio (OR) and 95%CI. Statistical significance was established at P < .05. The SPSS (Statistical Package for Social Sciences) program for Windows version 15.0 software (SPSS Inc.; Chicago, Illinois, United States) was used for the statistical analysis.
RESULTSOf the total 63 167 persons whose clinical histories were reviewed, 48 744 were receiving antihypertensive drug treatment (77.2%). Of the patients being treated for hypertension, 6292 met the criteria for resistant hypertension, which represents an estimated prevalence of 9.9% (95%CI, 9.7-10.2) of all hypertensive patients and 12.9% (95%CI, 12.6-13.2) of those treated. The estimated prevalence of resistant hypertension in all hypertensive patients was higher according to age; the prevalence was 4.4% (4.8% in men and 3.7% in women) in patients < 50 years and was 12.9% (11.7% in men and 13.4% in women) in patients > 80 years (P < .001). Table 1 shows the general characteristics according to the presence or absence of resistant hypertension. Compared with the 42 452 patients who did not have resistant hypertension, those who did were more frequently women (57%), were significantly older (mean age 70.5 vs 67.6 years), were less often smokers, had a higher prevalence of DM, hyperlipidemia and obesity, and a significantly lower glomerular filtration rate. The proportion of patients with associated comorbidity was also higher in those with resistant hypertension (P < .05).
General Characteristics of Patients With and Without Resistant Hypertension
| Resistant hypertension | No resistant hypertension | P value | |
| Patients, no. | 6292 | 42 452 | |
| Age, mean (SD), years | 70.5 (12) | 67.6 (14) | <.001 |
| Women, % | 57 | 55.7 | .007 |
| DM, % | 30.8 | 18.7 | <.001 |
| Hyperlipidemia, % | 38.2 | 35.3 | <.001 |
| Obesity, % | 52.2 | 40 | <.001 |
| Smoker, % | 4.6 | 5.8 | <.001 |
| BMI, mean (SD), kg/m2 | 31 (7) | 29.5 (21) | <.001 |
| SBP, mean (SD), mm/Hg | 144.8 (30) | 132.2 (33) | <.001 |
| DBP, mean (SD), mmHg | 80.9 (25) | 77.6 (17) | <.001 |
| Total cholesterol, mean (SD), mg/dL | 203.2 (48) | 207 (66) | <.001 |
| LDL cholesterol, mean (SD), mg/dL | 121 (35) | 127 (37) | <.001 |
| HDL cholesterol, mean (SD), mg/dL | 54 (15) | 55.6 (16) | <.001 |
| Triglycerides, mean (SD), mg/dL | 139 (88) | 129.6 (79) | <.001 |
| Creatinine, mean (SD), mg/dL | 0.98 (0.5) | 0.91 (0.5) | <.001 |
| GFR, mean (SD), mL/min/1.73 m2 | 77.5 (38) | 83.5 (55) | <.001 |
| BP <140/90mmHg, % | 19.2 | 61.7 | <.001 |
| Peripheral arterial disease, % | 2.4 | 1.8 | <.001 |
| Chronic kidney disease, %) | 7.5 | 3.4 | <.001 |
| Coronary heart disease, % | 15.8 | 7.7 | <.001 |
| Atrial fibrillation, % | 13 | 6.9 | <.001 |
| Stroke, % | 7.4 | 5.3 | <.001 |
| Heart failure, % | 7.3 | 3.3 | <.001 |
BMI, body mass index; BP, blood pressure; DM, diabetes melitus; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high-density lipoprotein; SBP, systolic blood pressure; LDL, low-density lipoprotein; SD, standard deviation.
In the whole population, 56.3% of patients had controlled BP (< 140/90mmHg) (54.8% in men and 57.4% in women; P < .001). Control was significantly better in patients < 65 years (58% vs. 55%; P < .001), in patients with CVD (60% vs 55%; P < .001), and in women > 65 years (60% vs. 55%; P < .001). A total of 19.2% of patients with resistant hypertension had BP controlled with 4 or more drugs.
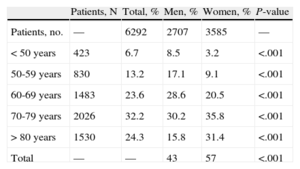
Table 2 shows the distribution of patients by age groups and sex. Notably, 67% of the women and 46% of the men with resistant hypertension were > 70 years (P < .001). Resistant hypertension was more frequent in men < 50 years and in women > 80 years (P < .001).
Distribution of Patients With Resistant Hypertension by Age and Sex
| Patients, N | Total, % | Men, % | Women, % | P-value | |
| Patients, no. | — | 6292 | 2707 | 3585 | — |
| < 50 years | 423 | 6.7 | 8.5 | 3.2 | <.001 |
| 50-59 years | 830 | 13.2 | 17.1 | 9.1 | <.001 |
| 60-69 years | 1483 | 23.6 | 28.6 | 20.5 | <.001 |
| 70-79 years | 2026 | 32.2 | 30.2 | 35.8 | <.001 |
| > 80 years | 1530 | 24.3 | 15.8 | 31.4 | <.001 |
| Total | — | — | 43 | 57 | <.001 |
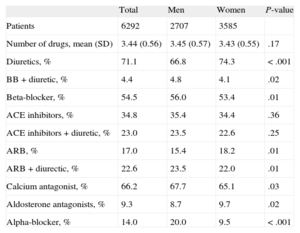
With regard to antihypertensive treatments, 59.6% of the patients were prescribed 3 drugs, 37.1% were prescribed 4 drugs, and 3.3% were prescribed 5 or more drugs. In patients with resistant hypertension, 100% were prescribed diuretics, 58.9% beta blockers, 66.2% calcium antagonists, and 97% inhibitors of the renin-angiotensin-aldosterone system. Table 3 shows the distribution of prescribed antihypertensive drugs in patients with resistant hypertension by sex, whether by single prescription or in combination. Diuretics and angiotensin-receptor blockers were more frequently prescribed for women, and beta-blockers, alpha-blockers, and calcium antagonists for men. In 9.3% of patients with resistant hypertension, aldosterone antagonists were prescribed. In 14% of patients alpha-blockers were prescribed (20% in men and 9.53% in women; P < .001).
Distribution of Prescriptions of Antihypertensive Drugs in Patients With Resistant Hypertension by Sex
| Total | Men | Women | P-value | |
| Patients | 6292 | 2707 | 3585 | |
| Number of drugs, mean (SD) | 3.44 (0.56) | 3.45 (0.57) | 3.43 (0.55) | .17 |
| Diuretics, % | 71.1 | 66.8 | 74.3 | < .001 |
| BB + diuretic, % | 4.4 | 4.8 | 4.1 | .02 |
| Beta-blocker, % | 54.5 | 56.0 | 53.4 | .01 |
| ACE inhibitors, % | 34.8 | 35.4 | 34.4 | .36 |
| ACE inhibitors + diuretic, % | 23.0 | 23.5 | 22.6 | .25 |
| ARB, % | 17.0 | 15.4 | 18.2 | .01 |
| ARB + diurectic, % | 22.6 | 23.5 | 22.0 | .01 |
| Calcium antagonist, % | 66.2 | 67.7 | 65.1 | .03 |
| Aldosterone antagonists, % | 9.3 | 8.7 | 9.7 | .02 |
| Alpha-blocker, % | 14.0 | 20.0 | 9.5 | < .001 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; BB, beta-blocker.; SD, standard deviation.
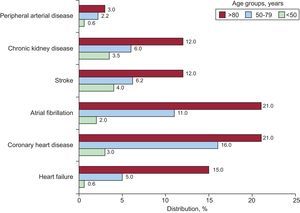
Notably, more than 50% of the patients with resistant hypertension > 80 years had some CVD (61.4% in men and 52.3% in women) (P < .001). Some 34.4% had one CVD, 13.7% had 2, 4.8% had 3, 1.7% had 4, 0.4% had 5 and 0.1 had 6. CVD was more prevalent in men in all age groups (P < .001). Data on CVD prevalence by age are shown in the Figure. The most frequently associated comorbidity was coronary heart disease and atrial fibrillation in patients > 80 years and stroke and kidney disease in patients < 50 years.
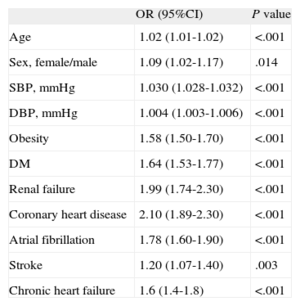
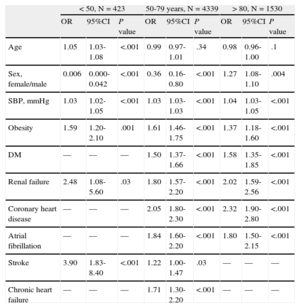
The results of the logistic regression analysis of the clinical characteristics associated with resistant hypertension are shown in Table 4. Because age and sex showed a statistically significant interaction (odds ratio = 1.007; 95%CI, 1.001-1.002; P = .02), a stratified analysis by age was carried out to check the effect of the variables in each age group. No differences were found in the stratified analysis by sex. Te results of the logistic regression analysis of the clinical characteristics associated with resistant hypertension by age are shown in Table 5. A total of 5.5% of patients were < 50 years, 69.8% were aged between 50 and 79 years and 24.7% were > 80 years. In patients < 50 years, resistant hypertension was associated with male sex (odds ratio female/male = 0.006; 95%CI, 0.000-0.042; P < .001), SBP, obesity, stroke, and chronic kidney disease (all P < .001). In those > 80 years, resistant hypertension was associated with female sex (OR female/male = 1.27; 95%CI, 1.08 to 1.10; P = .004), SBP, DM, obesity, chronic kidney disease, coronary heart disease, and atrial fibrillation (all P value < .001).
Multiple Logistic Regression Analysis With the Clinical Characteristics Associated With Resistant Hypertension
| OR (95%CI) | P value | |
| Age | 1.02 (1.01-1.02) | <.001 |
| Sex, female/male | 1.09 (1.02-1.17) | .014 |
| SBP, mmHg | 1.030 (1.028-1.032) | <.001 |
| DBP, mmHg | 1.004 (1.003-1.006) | <.001 |
| Obesity | 1.58 (1.50-1.70) | <.001 |
| DM | 1.64 (1.53-1.77) | <.001 |
| Renal failure | 1.99 (1.74-2.30) | <.001 |
| Coronary heart disease | 2.10 (1.89-2.30) | <.001 |
| Atrial fibrillation | 1.78 (1.60-1.90) | <.001 |
| Stroke | 1.20 (1.07-1.40) | .003 |
| Chronic heart failure | 1.6 (1.4-1.8) | <.001 |
95% CI, 95% confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; OR: odds ratio; DM, diabetes mellitus; dds ratio; SBP, systolic blood pressure.
Multiple Logistic Regression Analysis With the Clinical Characteristics Associated With Resistant Hypertension by Age 80 years
| < 50, N = 423 | 50-79 years, N = 4339 | > 80, N = 1530 | |||||||
| OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | 1.05 | 1.03-1.08 | <.001 | 0.99 | 0.97-1.01 | .34 | 0.98 | 0.96-1.00 | .1 |
| Sex, female/male | 0.006 | 0.000-0.042 | <.001 | 0.36 | 0.16-0.80 | <.001 | 1.27 | 1.08-1.10 | .004 |
| SBP, mmHg | 1.03 | 1.02-1.05 | <.001 | 1.03 | 1.03-1.03 | <.001 | 1.04 | 1.03-1.05 | <.001 |
| Obesity | 1.59 | 1.20-2.10 | .001 | 1.61 | 1.46-1.75 | <.001 | 1.37 | 1.18-1.60 | <.001 |
| DM | — | — | — | 1.50 | 1.37-1.66 | <.001 | 1.58 | 1.35-1.85 | <.001 |
| Renal failure | 2.48 | 1.08-5.60 | .03 | 1.80 | 1.57-2.20 | <.001 | 2.02 | 1.59-2.56 | <.001 |
| Coronary heart disease | — | — | — | 2.05 | 1.80-2.30 | <.001 | 2.32 | 1.90-2.80 | <.001 |
| Atrial fibrillation | — | — | — | 1.84 | 1.60-2.20 | <.001 | 1.80 | 1.50-2.15 | <.001 |
| Stroke | 3.90 | 1.83-8.40 | <.001 | 1.22 | 1.00-1.47 | .03 | — | — | — |
| Chronic heart failure | — | — | — | 1.71 | 1.30-2.20 | <.001 | — | — | — |
95%CI, 95% confidence interval; DM, diabetes mellitus; OR: odds ratio; SBP, systolic blood pressure.
This study provides recent data on the estimated prevalence, demography and clinical characteristics of resistant hypertension in routine clinical practice in a large group of hypertensive patients treated in primary care. A very important association was found with age, as demonstrated by the finding that 2 of every 3 women and 1 of every 2 men with resistant hypertension were > 70 years. Male sex, SBP, obesity, stroke, and chronic kidney disease were independently associated with resistant hypertension in persons < 50 years old. Female sex, SBP, obesity, DM, coronary heart disease, atrial fibrillation and chronic kidney disease were independently associated with resistant hypertension in patients > 80 years. More than 50% of the patients > 80 years with resistant hypertension had some CVD.
Our findings of the estimated prevalence of resistant hypertension in our study were similar to those found in previous observational studies.3–7 Similarly, the greater frequency of comorbidities and associated CVD are also consistent with previous observational studies3–6,25 and with a recent longitudinal analysis, reporting a 50% higher risk of cardiovascular events in patients with resistant hypertension over a 5-year follow-up.26 Nevertheless, the high prevalence found in clinical trials (15%-30%)8–10 is surprising, even though such studies presumably control all the biases. This disparity in the data reflects the distinct methodologies used and baseline differences between patients in clinical trials and community patients: in clinical trials, the population tends to be older with greater cardiovascular comorbidity than the general hypertensive population.27,28
The rate of BP control was similar to that reported in previous studies in the Spanish population, such as CARDIOTENS 2009 registry29 and was higher than in others, although the populations were different and, in the MERICAP study, BP control < 130/80mmHg in DM was considered.30,31 BP control in the whole population worsened as age increased, similar to the results reported by PRESCAP 2010.32
A notable finding was the apparently low consumption of aldosterone antagonists in our registry, which is considered an important factor in BP reduction in patients previously considered as resistant33,34; however, during the time period studied (2008), the use of these agents was uncommon among adults with resistant hypertension. There are few data that could be used for comparison. Data from the National Health and Nutrition Examination Survey from 2003 through 2008 show 3% to 15% prescription of aldosterone antagonists in resistant hypertension3,5 and recent studies of patients who were candidates for renal denervation have reported prescription from 17% in the Simplicity study to 82% in patients controlled in a multidisciplinary program on renal denervation in a Spanish setting.35,36
Data on the age and sex distribution of resistant hypertension are difficult to compare because there are no observational studies that analyze this point. Several clinical trials have reported beneficial results of treating hypertension in the elderly, but these studies have not provided clear guidance for selecting a specific BP value that could be used to diagnose hypertension or be used as a target for treatment and there is a relative lack of data for patients < 50 years and > 80 years.13,14,20 Recent studies such as the HYVET trial13 have reported the benefits of treating patients > 80 years, but these patients were generally healthier than those in the general population, only 11.8% had a history of CVD, and treatment was based on 2 drugs, aimed at achieving a target blood pressure of 150/80mmHg. There are also limited data on whether patients with initial SBP between 150 mmHg and 159mmHg would benefit from treatment.37 Nevertheless, the American College of Cardiology Foundation/American Heart Association 2011 guidelines recommend that values < 140 mmHg for those < 79 years of age are appropriate but for those > 80 years of age, 140 mmHg to 145mm Hg, if tolerated, can be acceptable.20 One might question whether it makes sense to set a BP goal of < 140mmHg and to include it as a criterion for resistant hypertension at such advanced ages, when reduction of BP to such a level may not even be desirable, especially in patients with associated CVD.38 On the other hand, the prevalence of orthostatic hypotension could be up to 20% in very old hypertensive patients and could be related to autonomous vascular disorders, chronic kidney disease, and cognitive impairment.39–41 Recent 2013 European Society of Hypertension/European Society of Cardiology guidelines mention that the recommendation of lowering SBP to < 150mmHg in elderly individuals with SBP > 160mmHg is strongly evidence-based, unlike the recommendation of SBP < 140 mmHg. However, at least in elderly individuals < 80 years, antihypertensive treatment may be considered at SBP values > 140mmHg and aimed at values < 140mmHg, if the individuals are fit and treatment is well tolerated.21
LimitationsOur study has the limitations characteristic of all cross-sectional and registry studies. Selection bias was minimized since we analyzed the entire population in the registry, but the results cannot be generalized because our population comes from a relatively limited area. Because data were not available, we were unable to analyze patient adherence to treatment, therapeutic inertia, pressure increasing drugs, or the adequacy of the maximum doses and thus our prevalence data may be overestimated, as in other observational studies.3–7 Moreover, we cannot be sure of the extent of the “white coat effect” which, according to the Spanish Ambulatory Blood Pressure Monitoring Registry, may be as high as 37.5% of resistant hypertension cases.6 Likewise, we were unable to analyze salt intake; reducing salt intake has been shown to lower BP values by as much as 20%,42 nor could we guarantee the exclusion of secondary hypertension, especially hyperaldosteronism, which is present in up to 10% of persons with resistant hypertension.43 Recent studies report that the common confounders in the office diagnosis of resistant hypertension, especially adherence to treatment, can be as high as 81%.44 The estimates provided here may overestimate the true prevalence of resistant hypertension.What our study does provide is an estimation of the prevalence of apparent resistant hypertension.
CONCLUSIONSOne in 4 patients with resistant hypertension is > 80 years. In patients < 50 years, resistant hypertension is associated with male sex, obesity, stroke, and kidney disease. In patients > 80 years, resistant hypertension is associated with female sex, obesity, DM, heart disease, and kidney disease. Although age and sex are nonmodifiable factors, guidelines on the management of resistant hypertension should bear in mind this distribution and the high prevalence of CVD in the very elderly people to avoid possible overtreatment and adverse effects. Although this is a descriptive study, it represents a huge sample in the Community of Madrid and adds new information that is potentially relevant both from an epidemiologic and clinical practice point of view.
CONFLICTS OF INTERESTNone declared.