Hospitalized patients with heart failure who are malnourished present a worse prognosis than those with an adequate nutritional status. It is unknown whether a nutritional intervention can modify the prognosis of these patients. The aim of this study is to assess the efficacy of a nutritional intervention on morbidity and mortality in hospitalized patients with heart failure who are malnourished.
MethodsPICNIC is a multicentre, randomized, controlled trial in which hospitalized patients with heart failure and malnutrition, as defined by the Mini Nutritional Assessment, are randomly assigned to conventional management of heart failure or conventional management of heart failure and an individualized nutritional intervention consisting of 3 points: optimization of diet, specific recommendations, and prescription, if deemed necessary, of nutritional supplements. A sample size of 182 patients for a maximum follow-up of 12 months has been estimated. The primary endpoint is time to death from any cause or rehospitalization because of heart failure. Analysis is by intention to treat.
ConclusionsPICNIC study will determine the prognostic impact of a nutritional intervention in hospitalized patients with heart failure who are malnourished.
Keywords
Epidemiologic studies consistently find that between 1% and 2% of the adult population in developed countries has heart failure (HF).1 This prevalence increases with age and reaches around 10% in individuals older than 70 years. In Spain, the PRICE study2 found figures that were even higher; the prevalence of HF was 6.8% in individuals over 45 years and 16% in those over 75 years of age. Despite the advances in treatment and the resulting improved prognosis,3 HF still is still associated with high morbidity and mortality.1,4 Study of HF outcomes has identified a number of significant prognostic factors,1,3,5 knowledge of which can help us optimize patient management in an attempt to modify the natural course of the disease. In recent years, some of the aspects that relate HF to malnutrition have been elucidated, although there are still many unknowns.
HF and nutritional state (NS) have a 2-way relationship. The catabolic state associated with HF6 can lead to deterioration characterized by progressive weight loss (cardiac cachexia), and this is associated with worse prognosis.7,8 On the other hand, different studies have shown, from a partial approach, the prognostic impact on a patient with HF of certain parameters often used to assess NS, such as body mass index,9 albuminemia,10 and cholesterolemia.11 However, no parameter in itself can give a precise assessment of NS, and so we need to take an integrative approach that includes the assessment of multiple and mutually complementary aspects.12,13 Such an approach forms the basis of different scores that aim to act as tools for the detection or diagnosis of malnutrition from an integrated perspective or, at least, a more complex one, including several parameters for assessing nutrition.12,14 Of the assessments, the Mini Nutritional Assessment (MNA) is particularly widespread in everyday clinical practice.15 This test has been validated to provide, in a simple and rapid manner, immediate information on the NS of the patient.16–18 The test has been used in a range of clinical contexts, both in hospitalized or institutionalized patients and outpatients.15 The prevalence of malnutrition in hospitalized patients assessed using the MNA is, in general, high, at around 25%.15 The prevalence reported obviously depends on the characteristics of the study population and, in some series of very elderly patients, malnutrition may be present in almost half.19 As with other methods of assessing nutrition, NS assessed by the MNA has prognostic significance. Thus, in hospitalized patients, malnutrition as determined by MNA is not only associated with a longer hospital stay,19–21 but also with a greater risk of readmission21 and greater mortality, both in hospital19 and in the long term.19,22 In patients admitted for HF, we have found that malnutrition according to the MNA score is present in 13% of the patients, associated with greater mortality, and an independent predictive factor in the medium and long term.23 Mortality among malnourished patients was 56%, compared to 11% among those adequately nourished within 12 months of discharge from hospital and after 25 months, 76% compared to 19% (log-rank test, P<.001; adjusted hazard ratio =3.75; 95% confidence interval [95%CI], 1.75-8; P=.001).23 At 12 months, 80% of the malnourished patients had died or been readmitted for HF compared to 30% of patients suitably nourished (log-rank test, P<.001).24
In view of the above, an important question is whether a nutritional intervention aimed at optimizing patients’ NS improves the prognosis of malnourished patients with HF. To date, few studies have assessed the effect of a nutritional intervention in patients with HF. Those that have been published have assessed the effect in general series, without considering the baseline NS of the patient, and so the effect reported is modest and limited to an improvement in the functional class or quality of life of the patient, without any impact on survival.25,26 In patients with cardiac cachexia, similar findings have been reported.27 The lack of robust clinical trials, with an appropriate design and focused especially on the subgroup of malnourished patients, means that there are currently no general recommendations on the type of nutritional intervention in patients with HF, with or without malnutrition, beyond those performed with respect to restrictions on sodium and liquid intake.3,26 The scientific basis for these recommendations is, moreover, limited.3 There is thus a need for clinical trials that provide information on this question. Therefore, it seems particularly relevant to apply a nutritional intervention in a patient with HF in whom malnutrition has already been detected, regardless of the underlying cause, which is usually multifactorial. We do not know whether a nutritional intervention can improve prognosis in these patients who, as mentioned above, have high morbidity and mortality.23,24
The aim of this study is therefore to test the initial hypothesis that application of a program of nutritional intervention tailored to malnourished patients hospitalized for HF could provide benefit in terms of morbidity and mortality.
The primary objective of the PICNIC study is to assess whether a nutritional invention in malnourished patients admitted to hospital for HF may provide benefit in terms of morbidity and mortality (expressed as all-cause death or readmission for HF).
The secondary objectives are to assess whether a nutritional invention in malnourished patients admitted to hospital for HF may have an impact on mortality, readmission to hospital (for HF or any other cause), quality of life, and NS of the patient.
METHODSStudy DesignThis was a multicenter, randomized, blinded, controlled study to analyze the outcome variables. The PICNIC study has been designed to assess the effects of a nutritional intervention on the morbidity and mortality of patients admitted to hospital for acute HF who, in addition, were malnourished.
The Hospitals Reina Sofía (Córdoba, Spain) and San Juan de la Cruz (Úbeda, Jaén, Spain) are participating in the study. Recruitment is expected to last 24 months. The maximum follow-up period stipulated for each patient is 12 months, and so the maximum study duration will be 36 months.
The study protocol has been evaluated and approved by the ethics committees of the provinces of the 2 participating centers.
The trial is registered in ClinicalTrials.gov (NCT01472237).
Study PopulationPatients aged over 18 years who are admitted for acute HF, whether chronic and uncompensated or of new onset, in a state of malnutrition (score on the MNA<17 points) are eligible for enrollment. The patients receive information both orally and in writing, and they agree to comply with the study protocol by signing the informed consent. Patients who require assistance for the activities of daily living or who have a certain degree of cognitive decline can participate in the study if they are institutionalized or have appropriate family support. In such cases, the informed consent should be signed by the person responsible for the patient, or his or her legal guardian.
Excluded from the study are pregnant women, patients with chronic renal failure in dialysis, patients already receiving nutritional treatment, patients with concomitant disease who, regardless of HF itself, have a life expectancy of less than 1 year, patients participating in other clinical trials, patients who undergo surgery or percutaneous coronary intervention during their hospital stay to correct the cause of acute HF, and patients whose clinical status means that it is impossible to perform the nutritional assessment as established in the study protocol or who do not provide their consent for such procedures.
Heart FailureDiagnosis of HF is established according to the recommendations of the European Society for Cardiology.28 Thus, clinical findings (signs and symptoms), chest radiograph, electrocardiogram, natriuretic peptides (NT-proBNP), and echocardiograms are analyzed. Diagnosis of HF with conserved systolic function is established when the left ventricular ejection fraction >50% in presence of signs and symptoms indicative of HF and substantial structural heart disease or indications of diastolic dysfunction.29 In the admission visit, demographic, clinical, laboratory, and treatment details will be collected (see supplementary material). General comorbidity will be assessed using the Charlson index.30 Glomerular filtration will be estimated using equation 7 of The Modification of Diet in Renal Disease (MDRD) study.31
Nutritional StateDiagnosis of malnutrition will be established according to the MNA score. This questionnaire assesses general nutrition and has been designed and validated to provide a simple and rapid assessment of NS of the patient. It includes 18 items distributed in 4 sections: anthropometry, general state, dietary aspects, and subjective assessment.16 A final score is obtained that classifies the subject into 1 of 3 possible categories: well nourished (≥24 points), at risk of malnutrition (17-23.5 points), and malnourished (<17 points). In addition, a full nutritional study will be performed using biochemical markers (albumin, prealbumin, transferrin, total cholesterol, and lymphocytes) and anthropometric variables (body mass index and tricipital skinfold as an indicator of fatty tissue and midarm muscle circumference as an indicator of muscle tissue).12,13 The anthropometric measures will be obtained using standard techniques.32 The weight and height of the patient will be recorded without shoes on and in light clothing on clinical scales with a height rod. The tricipital skinfold will be measured using Holtain skinfold calipers, with a precision of 0.2mm and a pressure of 10mg/mm2. For the measurement of midarm circumference, a tape measure will be used, calibrated in millimeters. The body mass index will be obtained using the formula: body mass index = weight (kg)/height2 (m2). The midarm muscle circumference will be obtained using the Jeliffe equation33: midarm muscle circumference = midarm circumference − (p × tricipital fold), expressed in centimeters.
Quality of LifeThe patient's quality of life will be assessed using the Minnesota Living With Heart Failure Questionnaire.34
Nutritional InterventionUsing a simple randomization process, 182 patients will be assigned to 1 of 2 groups: control or nutritional intervention. For this purpose, a randomization sequence has been generated and deposited in the secretariat of the Internal Medicine Department of Hospital Juan de la Cruz. The investigators are in contact with the staff of the secretariat by telephone. Patients in the control group receive conventional treatment for HF. They attend the cardiology appointment every 3 months or on request of the patient in accordance with his or her condition. Patients in the intervention group receive the same treatment for HF and follow-up by the cardiologist but they are also included in a personalized program for nutritional intervention, run by a physician specialized in nutrition and with the support of a dietary and nutritional technician. The nutritional intervention, which begins on admission, lasts 6 months and comprises the following points:
1. Diet Optimization. The patient follows a tailored diet according to the standards recommended for the general population with regard to calories and needs for macronutrients and micronutrients (nutritional goals),35,36 with the modifications considered appropriate in view of the patient’ comorbidities, particularly in the case of diabetes mellitus37 and kidney failure (Table 1).38
Nutritional Goals Considered When Designing the Diet of the Patients36
| Fat, % of total daily energy intake | 30-35 |
| Saturated fatty acids, % | 7-8a |
| Monosaturated fatty acids, % | 15-20 |
| Polyunsaturated fatty acids, % | 5 |
| Ω-6, g/day | 2 |
| Ω-3, mg/day | >200 |
| Cholesterol, mg/day | <300b |
| Carbohydrates, % of total daily energy intake | 50-55c |
| Proteins, % of total daily energy intake | 15-20d |
| Fiber, g/day | >25 |
| Salt, g/day | <5e |
| Vitamins and mineralsd: the recommended daily intake of the World Health Organization according to sex and age is used (micronutrients, when recommended, will not exceed the maximum tolerable intake)36 | |
Before starting the PICNIC study, the recommendation to restrict the intake of salt for patients with heart failure was not set at a particular level.28 For the prevention of cardiovascular disease, since 2003, the World Health Organization recommends a diet with a daily salt content < 5 g,36 and so this is considered as the recommendation when drawing up the diet of the patients in the PICNIC study. This recommendation is in line with the current recommendation of the European Society of Cardiology for patients with heart failure.3
2. Specific Recommendations. Specific recommendations are those aimed at tackling lack of appetite and overcoming the problems faced by the patient with regards other aspects related to the digestive process, such as chewing or swallowing disorders, nausea, dyspepsia, or intestinal transit disorders (Table 2).
Some of the Specific Recommendations for an Appropriate Intake
| Loss of appetite |
| Different foods and methods of preparation are proposed |
| Prepare the food according to the preferences of the patient |
| Eat small quantities of food at different times during the day (5 or 6) |
| Preferably, drink liquids between meals rather than at mealtimes |
| Soft foods are better tolerated |
| Concentrate the calorie count, such that an appropriate amount of energy can be administered with little food |
| Problems chewing |
| Cook the food more thoroughly |
| Use soft foods or blend |
| Problems swallowing |
| Blended food |
| Administration of liquids with thickeners |
| Nausea or dyspepsia |
| Prepare the food according to the preferences of the patient |
| Eliminate food that triggers symptoms |
| Preferably use soft foods |
| Eat small quantities of food at multiple times during the day (5 or 6) |
| Intestinal transit disorders |
| Modify the fiber content of the diet |
3. Nutritional Supplements. Nutritional supplements will only be used at the discretion of the physician when the nutritional goals are considered unattainable by following conventional dietary advice. The supplement used in each case will be that indicated by the physician in accordance with the clinical situation of the patient and his or her comorbidities. The supplement will also be adapted to the particular needs of the patient in order to attain the established nutritional goals.
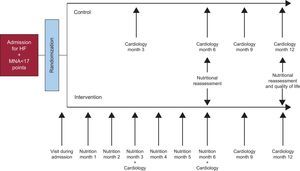
In the first visit, the patient will be required to provide details of his or her food intake through a 24-h recall. This will allow baseline analysis of the content of macronutrients and micronutrients in the patient’ diet by means of the Dietsource® computer program for nutritional analysis. The same program will be used to elaborate the tailored diet and to analyze the content of the diet at the end of the intervention. The data collected on the content of macronutrients and micronutrients in the patient’ diet before and after the nutritional intervention will be averaged over 3 days. The nutritional intervention begins on admission. After discharge from hospital, the patient will undergo follow-up each month for 6 months in a specific nutritional visit that the nutritional intervention lasts. In these visits, the appropriate recommendations will continue to be made to satisfy the demands of the patient regarding the aspects mentioned above. Adherence to the diet will be assessed through a reminder of ingestion at 24h and the patients will be reminded of the need to comply. The study design is shown schematically in the Figure.
EvaluationThe data on morbidity and mortality will be collected at the end of follow-up (12 months) either by means of a direct interview with the patient or his or her family members, either over the telephone or by extraction from the digitalized medical records available to the participating centers.
For evaluation of the primary endpoint, the main outcome variable of the study will be time to a composite endpoint of all-cause death or readmission for HF. Among the causes of death, distinction will be made between death of a cardiovascular origin (sudden death, death due to progression of HF, and other) and death of a noncardiovascular origin. Admission due to HF will be considered hospital stays of more than 24h due to the onset of signs and/or symptoms of HF decompensation (to this effect, hospital admission will also be considered as a stay of more than 24h in the emergency room).
The following secondary endpoints will be studied: time to all-cause death, time to admission for HF, and time to admission for any cause. In addition, we will assess whether the nutritional intervention has led to changes in patient quality of life or NS. To do this, the quality of life of the patients will be reassessed using the Minnesota Living With Heart Failure Questionnaire after 12 months of follow-up. The NS will be assessed by means of the MNA and biochemical and anthropometric nutritional parameters at 6 and 12 months (Figure).
Statistical AnalysisFor the comparison between groups, the χ2 test is used for qualitative variables. In the case of quantitative variables, the Student t test or Mann-Whitney U test will be used for parametric and nonparametric variables, respectively. To assess the effect of the intervention on the different quantitative variables, an analysis of covariance (ANCOVA) was performed. This yields the Kaplan-Meier survival curves, which are then compared using the log-rank test. The effect of the intervention will be expressed as a hazard ratio (and its 95% confidence interval), obtained from the Cox univariate regression analysis, considering time to event as the dependent variable and as the only factor in the group randomization model. In addition, the clinical impact of the intervention will be assessed by calculating the number of patients needed to treat. The data will be analyzed according to the intent-to-treat principle. Two interim analyses are contemplated to assess the primary outcome measure of the study, and the level of significance in both cases is established at P<.001.
All statistical analyses will be performed with the SPSS® program, version 15 (SPSS Inc.; Chicago, Illinois, United States).
Sample SizeFor the calculation of sample size, a composite event of all-cause death and readmission due to HF at 1 year of follow-up was used, based on data from the preceding observational study.23,24 For an estimated incidence of events in the control group of 80%, a relative reduction of 25% with the intervention, a power of 80%, and an alpha error for the 2-tailed test of .05, a total of 91 patients per group will be required, assuming that 10% of those initially enrolled will be lost to follow-up, in order to detect the desired effect.
Administrative AspectsThe PICNIC study will be conducted under the auspices of a scientific committee responsible for the design of the protocol and the appropriate execution. In addition, this committee is responsible for building the database and analyzing the results. Although the initial project contemplated starting recruitment in November 2011, administrative problems beyond the control of the investigators delayed the start of the study until March 2012. Currently, the recruitment rate is below that estimated initially, due above all to a decrease in the number of patients admitted to hospital for HF compared to the rate foreseen. As a result, the recruitment phase has been extended to 24 months instead of the 15 months initially foreseen (ClinicalTrials.gov: NCT01472237).
The PICNIC study is funded by the Spanish Society of Cardiology as a 2011 Project of the Spanish Society of Cardiology for Clinical Investigation in Cardiology.
DISCUSSIONCurrently, there are no specific nutritional recommendations for patients with HF.3,26 Likewise, for the subgroup of malnourished patients with HF, there are no recommendations even though morbidity and mortality are high.23,24 In view of the above, the PICNIC study was conceived to answer the question whether an intervention aimed at improving the NS of a malnourished patient with HF as an add-on to convention treatment for HF can help modify the natural course of the disease in these patients. Previously, the few studies that have assessed the effect of a nutritional intervention in patients with HF have shown a small effect, but these are limited to changes in body composition, functional class, or quality of life.25–27 The most important contributions of the PICNIC study are, on the one hand, that the intervention is performed on a selected series of patients with HF, that is, malnourished patients, and on the other, that the main objective of the study is expressed in terms of morbidity and mortality.
CONCLUSIONSMalnourished patients admitted to hospital for HF have a much higher morbidity and mortality than those with adequate NS. We do not know the prognostic effect of a nutritional intervention in this group of patients. The PICNIC study is a randomized, controlled clinical trial to assess whether a nutritional invention in malnourished patients admitted to hospital for HF may provide benefit in terms of morbidity and mortality. The first patient was randomized in March 2013 and it is foreseen that recruitment will last 24 months. The results of the PICNIC study will show the impact on prognosis of a nutritional intervention in malnourished patients admitted to hospital for HF.
CONFLICTS OF INTERESTNone declared.