INTRODUCTION
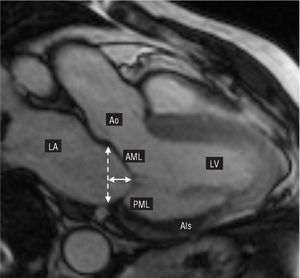
Ischemic mitral regurgitation (IMR) is a complex disease with a poor prognosis. Restricted mitral leaflet motion during the end-systolic phase (Carpentier's type IIIb dysfunction, Table 1), which is more accentuated in the posterior leaflet, has been described as the leading cause of mitral regurgitation of ischemic origin.1 After severe myocardial damage, the left ventricle undergoes a change in its elliptical morphology acquiring a more spherical shape.2 The increase in the ventricular sphericity index leads to an apical and lateral displacement of the papillary muscles (particularly in the posteromedial papillary muscle), thus creating an imbalance between the forces of traction and coaptation in the mitral subvalvular apparatus. Displacement of the papillary muscles involves apical tenting of the valve leaflets. This phenomenon stops the free edges of the leaflets from reaching the physiological annular plane (Figure 1), thus causing mitral regurgitation.
Figure 1. Cardiac magnetic resonance imaging (vertical long axis, 2-chamber view, maximum systolic pressure). The ischemic area (AIs) involving the posteromedial papillary muscle was calculated (courtesy of Dr Javier Sanz). LA indicates left atrium; Ao, aorta; continuous line, tenting height; discontinuous line, physiological plane of coaptation and mitral annular diameter; AML, anterior mitral leaflet; LV, left ventricle; PML, posterior mitral leaflet.
Ischemic papillary muscle dysfunction is compounded by the creation of excessive tension in the chordae tendinae and impedes the correct coaptation of the mitral leaflets, further worsening the grade of mitral regurgitation. In IMR, postischemic ventricular remodeling is the main factor catalyzing mitral dysfunction; nevertheless, dilatation and later annular deformation -- the result of the lack of valvular coaptation -- are frequently associated with type-IIIb dysfunction.
Three-dimensional echocardiography has refined the analysis of the valve leaflets leading to higher levels of detection and better quantification of IMR. Kwan et al3 corroborated a concept previously postulated by our group, demonstrating that mitral valve deformation follows an asymmetrical pattern along its commissural diameter. They also confirmed that in patients with IMR there is marked tethering of both leaflets at the posteromedial edge (segments A3 and P3), whereas tenting was less pronounced in the segments at the anterolateral edge (segments A1 and P1). Furthermore, in an ovine model of chronic ischemic mitral regurgitation, Gorman et al4 showed that all the segments of the mitral annulus, including the anterior segments, are asymmetrically dilated, especially in the posteromedial segment.
To date, the surgical treatment of choice for patients presenting IMR is mitral valve annuloplasty, employing a great variety of surgical techniques in combination with myocardial revascularization.5
Recent findings, some already mentioned, indicate that most of the surgical techniques used during the last decade may not be the optimal treatment in preventing the long-term appearance of residual or recurrent mitral regurgitation (MR), observed in 10%-15% of patients during clinical follow-up.6-8
A better understanding of the pathophysiological mechanism of type-IIIb IMR has led to the development of a specific ring for this clinical entity: the Carpentier-McCarthy-Adams IMR Etlogix (CMA-E) annuloplasty ring.9 This new prosthetic ring not only corrects the general dilatation of the native valve, but also has the aim of correcting the asymmetrical dilatation and tethering of segment P2 and P3. The resulting increase in leaflet coaptation surface should lead to more durable long-term functionality. In this study, we describe our initial clinical experience with the new prosthetic mitral ring in patients with type-IIIb IMR.
METHODS
The study included 40 consecutive patients who underwent interventions for type-IIIb ischemic mitral regurgitation using the new CMA-E prosthetic mitral ring (Edwards LifeSciences, Irving, California, USA) between December 2003 and June 2005. Patients who had already undergone surgery or who required additional tricuspid valve surgery were also included in the study group. A database was used to retrospectively identify all the patients. The clinical record of each patient was reviewed to obtain additional information. The study was approved by the ethical research committee of our institutional review board and the research period established following the international recommendations on clinical research (HIPPA) according to the Declaration of Helsinki developed by the World Medical Association (1975).
All the patients underwent preoperative transthoracic echocardiographic study; restricted valve leaflet motion during the end-systolic phase was confirmed in all cases (type-IIIb IMR, Carpentier Functional Classification). The severity of MR was prospectively assessed in all the patients based on regurgitant jet area (color Doppler ultrasound) and regurgitant volume quantified by semiquantitative Doppler ultrasound and 2-dimensional echocardiography. The grade of MR was quantified from 0 to 4+ (0, none; 0.5+, trace; 1+, mild; 2+, moderate; 3+, moderate to severe; and 4+, severe).10
Furthermore, the geometry of the mitral apparatus was evaluated by measuring the mitral annular diameter, mitral valve tenting height (distance between the mitral leaflet coaptation point and its physiological plane of coaptation) and mitral valve tethering area.
Carpentier-McCarthy-Adams IMR Etlogix Annuloplasty Ring
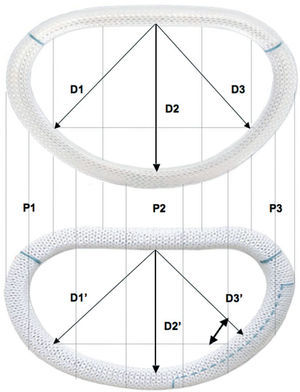
Two basic concepts came together when creating the new ring: reducing the mitral annular diameter using an undersized ring, and correcting the asymmetrical annular deformation of the native mitral ring and thus return it to its characteristic saddle shape. This new design leads to an increase in the valve leaflet coaptation surface area by reducing the anteroposterior dimension (D2 vs D2', which remains invariable during the entire cardiac cycle due to its rigid titanium core), and the septolateral dimension (D3 vs D3'). Thus, there is an annular reduction of 1 size for the anteroposterior dimension (D2') and of 2 sizes for the septolateral dimension (D3') compared to the Carpentier-Edwards Physio ring (Figure 2). This makes it possible to select the size of the prosthetic ring based on the dimensions of the anterior mitral leaflet (calibrated using a standard Carpentier-Edwards sizer), which enables valve remodeling with optimal coaptation in segments P2 and P3.
Figure 2. Comparison between the classic Physio (upper) and the new CMA-E ring (lower). Differences between the dimensions outlined within the 2 rings were calculated: septomedial dimension (D1 and D1'), anteroposterior dimension (D2 and D2'), and septolateral dimension (D3 and D3'). D2' is 1 size less than D2, and D3' is 2 sizes less than D3.
Statistical Analysis
All values are expressed as mean (SD) and quantitative variables as median and interquartile range (IQR). The Student t test was used for paired data and to compare echocardiographic measurements before and after the surgical procedure. Discrete variables are presented as percentages and compared using χ2. P<.05 was considered statistically significant. The SPSS software package (version 15.0, SPSS., Chicago, Illinois, USA) was used for the statistical analysis.
RESULTS
Table 2 presents the clinical characteristics of the patients. The study population included 30 men (75%) and 10 women (25%). The mean age was 68 (10) years. Mean ejection fraction (EF) was 39% (14%) (range, 14%-64%). The grade of coronary disease among patients was distributed as follows: 9 (22%) patients presented 1-vessel coronary disease; 14 (35%), 2-vessel coronary disease; and 17 (43%), 3-vessel coronary disease.
The preoperative echocardiographic MR grade among patients was distributed as follows: 28 (70%) patients presented grade 4+ MR and 12 (30%), grade 3+ MR.
Surgical Procedures
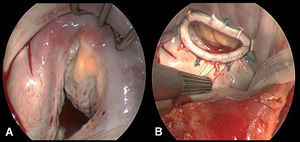
Myocardial revascularization was performed in 27 (68%) patients. The mean number of coronary grafts was 2.7 (1.3). Before mitral repair a modified Maze procedure was performed in 9 (22%) patients with a history of atrial fibrillation, and a cryoablation technique used for tissue ablation (Cryocath Technology, Pointe-Claire, Canada).11 Intraoperative exploration confirmed previous echocardiographic findings in all cases. The CMA-E prosthetic mitral ring was successfully implanted in all patients; it was fixed to the native valve using interrupted mattress sutures (12-15 stitches). The sutures were overlapped at P2, P3, and the posteromedial commissure, with the aim of releasing excess tension and thus avoiding the potential risk of annular dehiscence. Ring size was decided after measuring the commissural distance and anterior leaflet surface area, using a standard Carpentier-Edwards sizer. After it was implanted and fixed to the native valve, the valve was examined to rule out any possibility of residual leakage by injecting pressured saline serum into the ventricular cavity, thus confirming that the coaptation area was good (>=8 mm). The line of coaptation was typically asymmetrical due to extensive previous tenting in segments P2, P3, and in the posteromedial commissural area (Figure 3). Table 3 shows the prosthetic ring sizes used and concomitant procedures followed. Mean cardiopulmonary bypass and aortic clamp times were 162 (70) min and 115 (50) min, respectively.
Figure 3. A: intraoperative view prior to mitral valve annuloplasty. B: intraoperative view of the implanted CMA-E ring.
Morbidity and Mortality
Mortality during hospital stay or 30 days postprocedure was 2.5% (n=1). The cause of death was multiorgan failure due to prolonged low cardiac output syndrome. In the postoperative period there was 1 case (2.5%) of stroke, 1 (2.5%) of kidney failure (creatinine >2.5 mg/dL for more than 7 days or need for dialysis), 4 (10%) respiratory failure (need for mechanical ventilation for more than 72 h), and 1 (2%) sternal wound infection. Three patients (7%) required a permanent pacemaker. There were no cases of annular dehiscence or early endocarditis.
Median hospitalization time was 11 (IQR, 7-15) days. Table 4 shows postoperative events.
Echocardiographic Findings
Echocardiographic findings are shown in Figure 4. Mitral valve regurgitation grade was significantly reduced in all patients (3.8 [0.4] and 0.3 [0.3]; P=.001). Mitral valve geometry values were also significantly reduced after surgery: mitral valve annular diameter (3.2 [0.8] cm to 2.3 [0.05] cm; P=.001), tenting height (0.6 [0.2] cm to 0.4 [0.1] cm; P=.001), and tethering area (1.2 [0.4] cm to 0.6 [0.3] cm; P=.001) (Figure 5). There were no significant changes in ejection fraction (39% [14%] to 40% [11%]; P=.631) and no case of systolic anterior motion occurred. At hospital discharge, 36 (90%) patients did not present postoperative residual MR and only 3 (10%) presented residual mild MR.
Figure 4. Echocardiographic changes in mitral regurgitation grade during the study period. IMR indicates ischemic mitral regurgitation.
Figure 5. Comparison between preoperative and postoperative values associated with mitral valve geometry variables (mitral valve annular diameter, tenting height, and tethering area) and ejection fraction.
Clinical and Echocardiographic Follow-Up
Clinical and echocardiographic follow-up was conducted in 97% (38/39) of the patients who underwent successful surgical intervention and were discharged; 2 (8%) late deaths occurred at 7 months and 12 months. Echocardiographic follow-up ≥15 months (range, 15-34 months) was conducted in 36 (92%) patients. The echocardiographic study demonstrated the absence of MR in 22 (61%) patients, mild MR in 13 (36%), and moderate MR in 1 (3%); the rate of recurrence of MR was 3%.
DISCUSSION
In this observational study, we describe our first clinical experience with the new CMA-E prosthetic mitral ring designed for the surgical treatment of patients with type-IIIb IMR. The new ring is effective in correcting this type of IMR and offers promising initial clinical results. Clinical and echocardiographic follow-up in 97% of the patients demonstrated the reliability and durability of the new ring and indicated an MR recurrence rate of 3% (n=1). During the study period, no complications associated with the prosthetic ring were observed, such as annular dehiscence, early endocarditis, or thromboembolic events.
A Specific Ring for a Specific Condition
Many studies have been conducted in an attempt to better understand the causal pathophysiological mechanisms of IMR, thus leading to the development of new surgical techniques and devices aimed at treating this complex valve lesion.12-14 The first studies addressing this issue established several valve repair techniques, such as suture annuloplasty (de Vega-type mitral annuloplasty), the use of cardiac rings, or the implantation of partial bands of variable flexibility, all of which are of limited durability and lead to residual MR and its reappearance in the short-term.15-17 Hausmann et al16,18 reported a series of 140 patients who underwent suture annuloplasty for IMR with an incidence of residual MR (>=2+) of 28%. In a report from the Cleveland Clinic Foundation group, Bhudia et al19 demonstrated that posterior annuloplasty and the implantation of a flexible partial band in combination with edge-to-edge repair was associated with an MR recurrence rate of 30% at 18-month clinical follow-up.
In a clinico-anatomical study of the heart human, Hueb et al20 contributed interesting data on mitral valve geometry in patients with IMR, demonstrating that in patients with ischemic cardiomyopathy or dilated cardiomyopathy, annular deformation was present in all the ring segments, including the anterior ones. These findings were later confirmed by Gorman et al21 in an ovine model of chronic IMR.
They verified that dilatation of the anterior and posterior mitral ring segments, together with asymmetrical ventricular dilatation, was the cause of tethering, and thus, of mitral regurgitation. The experience of several research groups has shown that annuloplasty using flexible partial bands may not be the optimal treatment technique for patients with type-IIIb IMR. We consider that these types of prosthetic ring do not completely remodel the native valve and, in particular, neglect the insertion area of the anterior mitral leaflet.
Similar findings have been described regarding annuloplasty using complete flexible rings in patients with IMR. Tahta et al22 analyzed long-term outcomes in 100 patients who had undergone combination surgery (mitral valve annuloplasty and coronary artery bypass grafting) using a Duran flexible annuloplasty ring. Over a mean clinical follow-up time of almost 36 months, 29% of the patients presented moderate (2+) recurrent mitral regurgitation. In our opinion, flexible rings would not succeed in stabilizing the native valve during the systolic phase; they reduce the intercommissural distance of the native valve, but have a limited impact on its anteroposterior diameter, a critical parameter in these types of patient. On the other hand, annular remodeling using a rigid or semirigid ring -- a concept supported by the Miller23 research team -- would reestablish the size and shape of the mitral ring, which also contributes to a significant reduction in anteroposterior diameter.
In this line, in 2004, Bax et al24 described the efficacy of restrictive annuloplasty using a Carpentier-Edwards Physio ring (Carpentier rigid ring) for the treatment of type-IIIb MR. In a series of 51 patients with grade 3+/4+ MR, the authors reported the absence of MR after a mean clinical follow-up time of 2 years.
Restricted valve leaflet motion in type-IIIb IMR causes a reduction in the leaflet tissue available for optimal coaptation; thus, we believe that an aggressive reduction in anteroposterior diameter would provide a suitable coaptation surface area. In the mid-1990s, Bolling et al25 introduced the concept of mitral reconstruction using an undersized prosthetic ring in order to reduce the incidence of residual or recurrent MR. Currently, this concept is widely accepted by most surgeons and is applied in mitral valve annuloplasty in patients with type-IIIb IMR. Furthermore, the excellent results described by Bax et al24 support both this and the use of undersized prosthetic rings.
CONCLUSIONS
The new CMA-E prosthetic mitral ring represents another advance in annuloplasty mitral valve repair. This prosthetic ring combines the principle of overcorrection with repair of the asymmetrical annular deformation in type-IIIb dysfunction. Its morphology provides an increase in valve leaflet coaptation surface area due to the reduction in anteroposterior diameter. Its asymmetrical three-dimensional design, and the reduced curvature in segments P2 and P3, lead to better tenting correction. Our study shows that the ring is very effective in correcting type-IIIb IMR. Echocardiographic results support the efficacy of the repair over a clinical follow-up time of 15 months to 34 months. However, more clinical series including a greater number of patients are needed and even longer clinical follow-up times to confirm the durability of this reconstruction technique.
ABBREVIATIONS
CMA-E: Carpentier-McCarthy-Adams IMR Etlogix
EF: ejection fraction
IMR: ischemic mitral regurgitation
IQR: interquartile range
MR: mitral regurgitation
See editorial on pages 1122-6
Project financed by the Department of Cardiothoracic Surgery.
Correspondence: Dr. J. Castillo/Dr. F. Filsoufi,
Research Fellow, Department of Cardiothoracic Surgery,
Mount Sinai Medical Center,
1190 Fifth Avenue, New York, NY 10029-1028 USA
E-mail: javier.castillo@mountsinai.org
Received March 18, 2007.
Accepted for publication June 29, 2007.