Keywords
INTRODUCTION
Sleep apnea-hypopnea syndrome (SAHS) is a condition defined by excessive somnolence, and cognitive-behavioral, respiratory, cardiac, metabolic, and inflammatory abnormalities, secondary to repeated episodes of upper airway obstruction during sleep.1 Several epidemiological studies carried out in the United States and Europe have documented the elevated prevalence of this disease, which affects 4%-6% of men and 2%-4% of women in the adult, middle-aged population.2,3 Moreover, the prevalence of SAHS clearly increases with age.4
Because of the pathophysiological alterations produced in SAHS, cardiovascular repercussions develop by several mechanisms during the apnea episodes.5 In addition to the consequences of intermittent hypoxia, the decreases in intrathoracic pressures with failed attempts at inspiration during apnea lead to increased ventricular afterload, together with hypertensive crises due to catecholamine release.6,7
These alterations are the reason why SAHS is not only associated with hypertension, but also with other manifestations, such as heart failure,8 cerebrovascular events,9 and ischemic heart disease,10 and with an increase in arrhythmias and sudden death.11
In the light of these established associations, it seems logical that the prognosis of SAHS would be closely related with the incidence of cardiovascular events.12,13 Therefore, early detection of patients with a poor prognosis would be extremely useful.
Transthoracic echocardiography is a noninvasive tool used in daily practice that allows assessment of cardiovascular structure and function. Most echocardiographic studies in patients with SAHS show that systolic and diastolic left ventricular function recovers when they receive treatment.14
The Tei index is an easily determined echocardiographic parameter that allows evaluation of systolic and diastolic ventricular function. This index has proved to be useful for both the left and the right ventricle. Since it is virtually unaffected by heart rate and preload, normalization is not required, and it shows a correlation with the severity of the clinical symptoms and survival.15,16
Based on this background, we proposed a study to investigate whether echocardiographic, morphologic, or functional alterations attributable to SAHS are present at the time of the diagnosis and to determine the repercussions these alterations might have according to the severity of the disease.
METHODS
Patients
The study included 110 consecutive patients (age, 54 [13] years; 73.8% men), referred between June 2005 and June 2006 from a specialized pulmonology service. All patients had undergone prior assessment with a scale that evaluated the absence, presence, and frequency of the main symptoms related with SAHS, as well as secondary symptoms, such as lack of attention, lack of sexual desire, morning headaches, and enuresis. The symptoms that guide the diagnosis included snoring, number of apnea episodes, and daytime sleepiness, as assessed with the validated Spanish version of the Epworth scale.17 General anthropometric variables were also recorded, such as age, sex, neck circumference expressed in centimeters, and the body mass index (BMI), as well as comorbid conditions (known cardiovascular disease, hypertension under treatment, diabetes mellitus, cerebrovascular disease, smoking, alcohol consumption, and the use of sedatives). Patients were considered to have a high probability of being affected with SAHS if they presented at least 2 of the cardinal symptoms of the disease, that is, chronic snoring, episodes of apnea, and pathological daytime sleepiness (Epworth >10 points).
All patients had been diagnosed with SAHS by conventional respiratory polygraphy or polysomnography (carried out in 15 patients with inconclusive polygraphy results) and had an indication for continuous positive airway pressure (CPAP) treatment, according to the current practice guidelines.1
Initially, 110 patients were included in the study. The exclusion criteria were refusal to participate, patient under CPAP treatment, echocardiographic evidence of atrial fibrillation, bradyarrhythmia (<60 bpm), or tachyarrhythmia (>100 bpm), and known cardiac disease that was decompensated at the time of enrollment. Additionally, patients with nocturnal recordings considered invalid (less than 5 h of recording or technical problems with the instruments) and those without a sufficiently reliable echocardiographic window were excluded. Following application of these criteria, 103 patients were included in the study. The reasons for exclusion were refusal to participate (2 patients), atrial fibrillation (3 patients), and insufficient acoustic access (2 patients).
Two groups were formed based on the apnea-hypopnea index (AHI): SAHS was considered not to be severe if the AHI was <30 (group 1; n=36; 69.4% males, 54 [12] years) and severe if the AHI was ≥3018 (group 2; n=67; 76.1% males, 54 [13] years). All patients underwent a baseline Doppler echocardiography study before treatment administration.
Material and Methods
Sleep Test
All patients underwent respiratory polygraphy with an EMBLETTA® polygraph (ResMed, Spain), which is a multichannel system that only records cardiorespiratory variables, but has been duly validated versus conventional polysomnography.19 The recording channels used were: nasal airflow by a cannula/pressure transducer system, O2 saturation, and heart rate by digital pulse oximetry, snoring sounds, quantitation of the number of apnea episodes according to the patient´s position by a body position sensor, and thoracoabdominal movement by elastic rib cage, and abdominal bands with piezoelectric sensors. All recordings were reviewed manually by the same pulmonologist. Obstructive apnea was defined as an absence or a >90% reduction in the respiratory signal for >10 s in the presence of respiratory exertion, as detected by the rib cage and abdominal bands. Central apnea was defined as an absence or >90% reduction in the respiratory signal for more than 10 s in the absence of respiratory exertion as detected by the rib cage and abdominal bands. Mixed apnea was established when the respiratory event usually began with a central component and ended with an obstructive component.1 The AHI was considered to be the number of respiratory events (episodes of apnea or hypopnea) occurring per hour of recording in bed. The tests were considered valid when patients stated that they had experienced almost normal sleep for at least 3 h. SAHS was defined on the basis of AHI ≥10 and pathological daytime sleepiness (Epworth >10 points). Other recorded parameters that assess nocturnal hypoxemia were also included: mean SaO2 at night, minimum SaO2 attained, total recording time with SaO2 <90% (CT90), and percentage of obstructive apnea episodes. When the polygraph results were considered negative for the diagnosis of SAHS, but the clinical findings were highly suggestive of this condition, the patient was referred to a specialized sleep laboratory to undergo conventional polysomnography. For this study, patients were referred for echocardiography if they had an indication for CPAP, established when the AHI was ≥30 or ≥10 in the presence of other factors, such as pathological somnolence, cardiovascular risk factors, and known cardiovascular disease.1
Doppler Echocardiography Technique
Doppler echocardiography was performed by a single operator with a Sonos 5500® echocardiograph and a 2.5-MHz probe (Philips, Eindhoven, The Netherlands). Three consecutive measurements were taken for each parameter and the mean of the 3 measurements was used for the analysis. Measurements were recorded in midexpiratory apnea. The heart rate was determined by electrocardiography during the examination once the patient had "relaxed."
The various measurements of the cardiac chambers were taken in M mode, following the established practice guidelines.20 Ventricular systolic function was determined qualitatively in 2D mode for the right ventricle and according to the method of Teichholz for the left ventricle.
Transvalvular flow across the aortic, mitral, pulmonary, and tricuspid valves was examined by pulsed Doppler, with an analysis of the filling waves, time between the cessation and onset of mitral and tricuspid flow, and the left ventricular isovolumetric relaxation time. The right ventricular isovolumetric relaxation time was obtained by measuring from the R-wave peak on the electrocardiogram to cessation of pulmonary flow, and subtracting this value from the interval between the cessation and onset of tricuspid flow.16
The isovolumetric contraction times were obtained by subtracting the ejection time and isovolumetric relaxation time from the interval between cessation and onset of atrioventricular transvalvular flow.
The Tei index was calculated as the time interval between cessation and onset of atrioventricular transvalvular flow, minus the ventricular ejection time, divided by the ventricular ejection time.
Reproducibility
Intraobserver variability was calculated from the 2 most extreme values of the 3 measurements obtained for each echocardiographic parameter analyzed. Variability for the morphological variables was 1.8% (range, from 1.0% [left ventricular end-diastolic diameter] to 4.0% [right ventricular end-diastolic diameter]). Intraobserver variability for the left ventricle Doppler variables was 0.9% (range, from 0.2% [peak E-wave velocity] to 2.2% [E-wave deceleration time]). Variability for the right ventricle Doppler variables was 1.2% (range, from 0.68% [pulmonary ejection time] to 3.8% [right ventricular isovolumetric relaxation time]). A (integral) ∫κ-value of >0.8 was obtained in all cases.
Statistics
Continuous variables were compared with Student t test. The Levene test was taken into account for equality of variances. Categorical variables were compared with the χ2 test. A forward stepwise logistic regression analysis was performed to establish the factors predictive of SAHS severity. The correlation between SAHS severity and these variables was determined with Pearson´s correlation coefficient. A P value less than .05 was considered significant.
RESULTS
Clinical Characteristics of the Patients
The variables studied, expressed as absolute values and percentages for the entire series and for the SAHS subgroups established according to severity, are shown in Table 1.
In the comparison between the 2 groups, there was a significantly greater presence of active smoking and alcohol consumption, as well as a larger mean neck circumference in the group with severe SAHS. There was also a clear trend toward a higher BMI in this group.
Results for Doppler Echocardiography
The analysis of all patients showed normal values for the ventricular diameters and systolic function of both ventricles, with no statistically significant differences between the study groups except for the functional parameters; patients with severe SAHS presented shorter ejection times, as well as higher Tei indexes for both ventricles (Table 2).
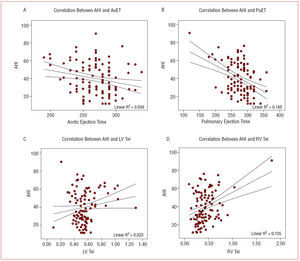
The correlation analysis (Table 3) showed significant differences for the variables corresponding to the right ventricular ejection time and Tei index (Figure).
Figure 1. Plots of the correlation between the apnea-hypopnea index (AHI) (y-axis) and: A) aortic ejection time (AoET), B) pulmonary ejection time (PuET), C) left ventricular Tei index (LV Tei), and D) right ventricular Tei index (RV Tei).
Multivariate analysis, adjusted for hypertension, BMI, smoking, and alcohol, disclosed that the only independent predictive variable for severity of SAHS was the pulmonary ejection time (odds ratio [OR]=0.98; 95% confidence interval [CI], 0.97-0.99; P=.01).
DISCUSSION
The cardiovascular repercussions of SAHS have been recognized for some time6-11 and it seems clear that the prognosis of this disease is linked to the incidence of cardiovascular events.12,13 Studies performed with noninvasive techniques, such as Doppler echocardiography,14 have assessed the prevalence of heart disease in these patients, as well as the response to CPAP treatment. Nevertheless, there is no in-depth study of the right chambers or the status of certain parameters that are reliable enough to allow early detection of cardiac repercussions in these patients. This was one of the objectives of the present investigation: to detect alterations in the morphological and functional (Tei index) echocardiographic parameters, and determine their usefulness as indicators of the severity of cardiac repercussions in a series of patients diagnosed with SAHS, in whom treatment had not yet been initiated.
The 2 groups established according to SAHS severity were quite homogeneous, with the exception of a more elevated use of tobacco and alcohol, and larger neck perimeter in the more severe cases. These factors, which contribute to the severity of SAHS, are also cardiovascular risk factors in the general population. In our series, however, only 2.9% of patients presented ischemic heart disease prior to the diagnosis of SAHS and we found no association between this factor and SAHS severity.
In the present series, the thickness of the interventricular septum and the posterior wall were within the normal range, with no differences between the 2 groups. In some studies the interventricular wall thickness, posterior wall thickness, and ventricular mass have shown a relationship with the severity of SAHS, particularly because of the link with the development of arterial hypertension and ventricular hypertrophy.14,21 The absence of differences in our series can be attributed to the equivalent distribution of hypertension in the 2 groups formed from our sample (47% in each), which was not the case in other studies.14,21
In the between-group comparisons, there were no significant increases in the right ventricular end-diastolic diameter, in contrast to the results of Shivalkar et al.14 In a study of 43 patients with severe SAHS and no pulmonary hypertension, these authors reported significantly larger ventricular diameters, which correlated with SAHS severity. Since the patients had no other diseases that could justify these findings, the authors attributed them to an increase of venous return together with septal movement toward the left,22 and to the transient presence of nocturnal pulmonary hypertension.23 Nevertheless, these changes have only been demonstrated during episodes of apnea,24 when there is associated pulmonary disease,25 or in cases of SAHS so severe that patients present diurnal hypercapnia.26
The study of left ventricular systolic function (diameters and ejection fraction) was normal in the 2 patient groups. The functional parameters, however, specifically the Tei index, were altered in both groups, with differences between these groups (greater involvement in the severe SAHS patients) and with respect to the reported data in healthy individuals.14,16 This can be explained by the shorter evolution time of the disease; the filling parameters for both ventricles had an atrial predominance in our 2 patient groups, a fact indicating that the diastolic function was affected, as has been described by other authors.27,28
The Tei index is a measure of myocardial performance and is related to ejection fraction, E-wave/A-wave ratio, systolic volume, peripheral resistances, and ventricular mass, and is independent of heart rate and blood pressure.29 In our series, the Tei index was increased in both ventricles with respect to the normal reported values.14,16 This increase was greater in the more severely affected SAHS group, with statistically significant differences as compared to the less severe group.
When the parameters comprising this index were analyzed separately, we found that the increases were produced by decreases in the aortic and pulmonary ejection times, which again, showed significant differences between the groups, with shorter times in the most severe group. In contrast to the findings of Arias et al,30 we did not observe increases in the isovolumetric relaxation times, which would also produce increases in the respective Tei indexes. Nonetheless, as has been reported by other authors,14 there was a good correlation between these parameters and SAHS severity in our patient population, particularly with regard to the right ventricle values. On multivariate analysis, the only variable that was independently associated with SAHS severity was the pulmonary ejection time.
The findings from this study suggest that the right ventricular Tei index and the pulmonary ejection time may be useful parameters for assessing ventricular function in the detection of anomalies caused by adaptation to the chronic stress these patients experience. A healthy ventricle has a lengthy ejection time, whereas a pathological one is characterized by gradual shortening of the ejection time with progression of the disease.31 This indicates that subclinical myocardial dysfunction can be detected through these parameters and that patients predisposed to develop heart failure can be differentiated from those that are not.32
This study has important implications for clinical practice because both the Tei index and pulmonary ejection time are easily and quickly calculated, and are sufficiently reliable to indicate the severity of SAHS, and to screen for silent myocardial dysfunction.
This is a cross-sectional study performed in patients diagnosed in a specialized SAHS service, with an indication for CPAP, and consecutively referred for echocardiographic study. One limitation of this research is the fact that no specific tests were performed to screen for ischemia. Nonetheless, given the absence of clinical symptoms, the fact that the ECG was not indicative, and the absence of segmental contractility alterations on echocardiography, we believe that such tests would not be a determinant factor in the study. In addition, it would have been desirable to have a control group matched for age, sex, and BMI for comparison with our patients. However, given the characteristics of the population required (BMI around 32 and no comorbid conditions), a control group with these features would be difficult to obtain.
Although this series contains a larger number of subjects than other published reports, we consider that the differences found would have been more pronounced if the less severe group had included more patients.
CONCLUSIONS
Myocardial performance is decreased in patients with SAHS. The Tei index (right and left ventricle) and the aortic and pulmonary ejection times are altered in this disease and are associated with SAHS severity. A decreased pulmonary ejection time indicates more severe disease.
ABBREVIATIONS
AHI: apnea-hypopnea index
BMI: body mass index
CPAP: continuous positive airway pressure
SAHS: sleep apnea-hypopnea syndrome
See editorial on pages 569-72
Correspondence: Dr. J.A. Moro López.
Enebro, 4, puerta 5. 46980 Paterna. Valencia. España.
E-mail: moro@uv.es
Received August 24, 2006.
Accepted for publication March 8, 2007.