Keywords
This work was partly financed by a Research Grant from the Fundación MAPFRE Medicina.
Received November 23, 2004.Accepted for publication July 8, 2005.
INTRODUCTION
Coronary flow depends on a complex multifactorial regulation mechanism that acts on the small vessels comprising the microcirculation.1 The state of the microcirculation is especially important in the context of revascularization after acute myocardial infarction (AMI) since it defines the quality of myocardial reperfusion once the lesion responsible for the infarction has been revascularized.2
The use of intracoronary sensor-tipped pressure and Doppler ultrasound guidewires in interventional cardiology makes it possible to apply the findings of coronary physiology to the resolution of clinical problems. In particular, these devices have been used to diagnose myocardial viability within AMI: appropriate occlusion pressure in the culprit vessel responsible for the AMI3 or progressive recovery of coronary flow reserve following initial impairment after an infarction4 have been associated with the preservation of myocardial viability. In addition, the analysis of phasic coronary flow characteristics with Doppler ultrasound guidewire has been related to the degree of myocardial perfusion in AMI,5 as well as to the recovery of postinfarction contractile function6 and, thus, to patient prognosis.7
Until now, the studies comparing the characteristics of coronary flow with myocardial viability have been done in the acute phase of the infarction. Our aim was to study whether the analysis of coronary flow continues to provide information regarding myocardial viability once the acute phase of the infarction has passed.
PATIENTS AND METHOD
Study Population
The patients were included consecutively from those sent to the interventional cardiology department of the Hospital do Meixoeiro, Galicia, Spain, from the hospital itself or from other centers without cardiac catheterization available, with the following criteria: signed informed consent where the study aims are described; first infarction with elevated ST segment of less than 1 month's evolution; 1-vessel disease with de novo lesion (50% stenosis) having undergone percutaneous coronary intervention (PCI) with successful angiography (<20% residual stenosis with TIMI 3 flow); serious abnormalities in segmentary contractility (akinesia) under contrast ventriculography; and, finally, absence of binary restenosis (stenosis <50%) at angiographic follow-up 4-6 months after PCI.
The final study population consisted of 19 patients from among the original 21 (2 patients could not be reassessed; 1 because of refusal and 1 having died of a non-cardiac cause).
Before carrying out PCI the severity of the lesion was evaluated by calculating the fractional flow reserve (FFR) with pressure guidewire and coronary flow reserve (CFR) with Doppler ultrasound guidewire. Following PCI, FFR was calculated again to assess the functional success of revascularization; likewise, CRF and coronary flow pattern were determined via Doppler ultrasound guidewire. Finally, at control follow-up (4-6 months post-PCI), CRF and flow pattern were again studied in the target coronary artery, as well as the extent of akinesia via contrast ventriculography.
Percutaneous Coronary Intervention
Revascularization was performed electively with direct stent implantation of according to the standard procedures followed in our department. The interventionist selected the type, diameter and length of the implanted stent. Doppler ultrasound guidewire was used to introduce the angioplasty devices.
Quantitative Angiography
Quantitative angiography (DLX, GE Medical System, Milwaukee, USA) of the culprit lesion was done before and after revascularization, as well as at follow-up. Intracoronary nitroglycerin (200 mg) was administered before angiography. The following parameters were measured: vessel reference diameter, minimum lumen diameter, percentage of stenosis, and lesion length.
On the other hand, ventricular contractile function was analyzed via contrast ventriculography in 30° RAO projection before PCI and at follow-up. Ejection fraction (Dodge method) and segmentary contractility in the culprit vessel territory were also quantified.
Segmentary contractility was analyzed via the centerline method. This method consists in the division of the cardiac silhouette into 100 segments defined by radii from the center of the cavity to its periphery. The end-systolic movement of each segment is expressed as fractional shortening according to its position at end-diastole. This value is then converted into standard deviation units (SD) from an average obtained from a normal reference population. Akinetic myocardium has a contractility of <2 SD of the reference average. In 30° RAO projection ventriculography, the myocardial silhouette lying between segments 10 and 65 corresponds to the vascular territory of the anterior descending artery; the vascular territory of the right coronary and circumflex arteries lies between segments 50 and 81. The state of regional contractility was expressed as the percentage of akinetic myocardial area compared to the total territory dependent on the culprit artery. The state of regional contractility was used at follow-up to assess the degree of viability of the affected vascular territory. The population was classified into 2 groups according to the extent of akinesia:
Preserved regional contractility (RC+), when the akinetic area was <30% of the myocardial area dependent on the culprit artery.
Impaired regional contractility (RC), when the percentage of akinesia was ≥30% of the affected vascular area.
Physiology of Coronary Flow
The physiology of coronary flow was analyzed with a pressure guidewire (WaveWire, Cardiometrics, Rancho Cordova, USA) and Doppler ultrasound guidewire (FloWire, Cardiometrics). These devices consist of 0.014-inch guidewires equipped with a sensor that measures intracoronary pressure (pressure guidewire) and also a 12-MHz Doppler ultrasound sensor that records systolic and diastolic blood flow velocity (Doppler ultrasound guidewire).8 Hyperemia was obtained via the intracoronary administration of a bolus of adenosine (Adenocor, Sanofi Winthrop, Barcelona) in a dose of up to 50 mg.
Assessment With Pressure Guidewire
By means of the pressure guidewire the FFR was calculated as the quotient between the mean pressure distal to the lesion and aortic pressure under maximum hyperemia, representing the coronary flow irrigating the vascular territory expressed as a percentage of that existing in the absence of the lesion.
Assessment With Doppler Ultrasound Guidewire
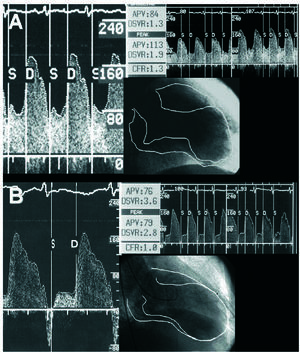
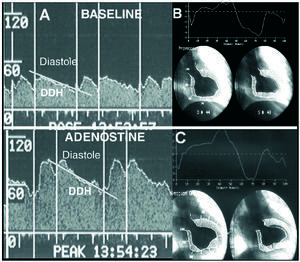
CFR was measured with this guidewire, and was taken to be the quotient between peak mean coronary flow velocity under hyperemia and at baseline. The following phasic coronary flow characteristics were also quantified: peak mean velocity of the diastolic component of coronary flow (cm/s); peak mean velocity of the systolic component of coronary flow (cm/s); and, finally, the deceleration halftime of the diastolic coronary flow velocity (ms), that is, the time taken for the maximum peak mean velocity to drop by half (Figures 1 and 2).
Figure 1. Coronary flow pattern after revascularization (A) and at follow-up (B) in a patient with contractile regional function which remained impaired in the territory of the anterior descending artery. The diastolic component of the flow shows an abrupt decrease in peak mean velocity.
Figure 2. Evaluation of coronary flow pattern in the right coronary artery in a patient with recovered contractile function in the lower wall at follow-up (B and C). Unlike the previous case, diastolic deceleration halftime is longer (A).
Statistical Analysis
This was an observational study made up of 2 cross-sectional studies where patients were assessed twice (at the beginning and at follow-up) and compared in relation to a criterion obtained at follow-up. Results were expressed as mean±SD. Data distribution was assumed to be non-normal given the low number of subjects. Thus, between-group comparisons were made via nonparametric tests (Mann-Whitney test for numerical variables and Fisher exact test for categorical variables). A Wilcoxon signed-rank test was used to analyze the evolution of variables within groups at follow-up. For each test a P-value <.05 was considered significant.
RESULTS
Classification of the Patients According to Regional Contractility at Follow-up. Clinical and Angiographic Characteristics
The 19 patients who finally completed the study were divided into 2 groups, according to the area of akinetic myocardium at follow-up (149±40 days): the RC+ group, consisting of 11 patients with an akinetic area of 8±11%; and the RC group, made up of the 8 remaining patients, with an akinetic area of 72±16%.
The 2 groups did not differ regarding clinical characteristics and hemodynamic situation during revascularization (blood pressure and heart rate). The only difference between groups was the location of the infarction, with a more frequent trend toward anterior location in the RC group (75% vs 27%; P=.05) (Table 1).
Regarding pre- and post-revascularization angiography, it is worth noting the greater percentage of lesions located in the anterior descending artery in the CR group (88% vs 27%; P=.01). There were no differences regarding angiographic and functional severity of the lesions (Table 2). At follow-up, angiography did not demonstrate differences in between-groups residual stenosis.
Functional Analysis of the Culprit Artery During Revascularization
Before revascularization, functional evaluation of coronary flow showed no differences between the CR+ and CR groups for FFR (0.51±0.24 vs 0.56±0.27) and CFR (1.3±0.4 vs 1.5±0.4).
After revascularization, RFF was ≥0.90 in 17 of the 19 patients studied (in the 2 remaining cases, 1 case per group, this parameter could not be determined due to technical problems). The average values of the FFR were identical in the CR+ and CR (0.95±0.04) patients.
After stent implantation, the CFR showed significant between-group differences (1.8±0.5 vs 2.3±0.5, respectively; P=.09) (Table 2).
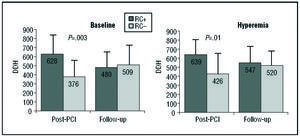
Regarding phasic coronary flow characteristics, diastolic deceleration half-time was significantly higher in CR+ patients (628±188 vs 376±131 ms; P=.004 at baseline, and 639±111 vs 426±176 ms; P=.01 under hyperemia) (Figure 3).
Figure 3. Comparison of diastolic deceleration halftimes in CR+ and CR group patients. In the immediate post-PCI analysis, patients in the CR+ group presented a significantly longer deceleration halftime, both at baseline and under hyperemia. These differences disappeared at follow-up. RC indicates regional contractility; DDH, diastolic deceleration halftime.
Functional Analysis of Coronary Flow at Follow-up
There were no differences between groups regarding the functional analysis of coronary flow at follow-up, nor in CFR (2.3±0.5 vs 2.3±0.7, for the CR+ and CR groups, respectively), nor for the phasic coronary flow characteristics at baseline and under hyperemia (Figure 3).
Evolution of Functional Flow Parameters at Follow-up
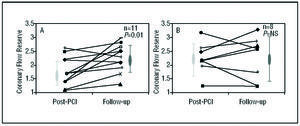
The characteristics of coronary flow in patients with preserved regional contractility at follow-up evolved differently to those in patients with persistent impaired contractility (Table 3). There was a significant increase in CFR between the value obtained after stent implantation and at follow-up in CR+ patients (1.8±0.5 vs 2.3±0.5, respectively; P=.01); such changes did not occur in CR patients (2.3±0.5 vs 2.3±0.7) (Figure 4). The improvement in CFR in the CR+ group at follow-up was due to the increase in peak mean velocity values under hyperemia in relation to the post-PCI study, and reached significance only for the diastolic component (86.6±42.1 vs 64.1±23.5 cm/s; P=.03).
Figure 4. Evolution of coronary flow reserve between the initial post-PCI evaluation and follow-up in patients with (A) and without (B) myocardial viability.
The diastolic deceleration half-time values evolved differently in both groups of patients, albeit in opposite ways, which explains the homogeneity of the data for both groups at follow-up. Thus, at baseline, the diastolic deceleration half-time increased significantly in the CR group (376±131 vs 509±198 ms; P=.03) and decreased in the CR+ patients (628±188 vs 480±143 ms; P=.01.
DISCUSSION
The main finding in this study is that, in the first month after an AMI, coronary flow in the culprit artery analyzed with Doppler ultrasound guidewire presents different characteristics in patients with and without viable myocardium in the affected vascular territory. Subsequently, these differences disappear.
Pattern of Coronary Flow in the Culprit Artery After Revascularization
The characteristics of phasic coronary flow velocity in the culprit artery after its revascularization within the context of primary angioplasty has been previously studied.6,7 Two differential coronary flow patterns indicative of myocardial perfusion quality have been described, as well as the degree of recovery of contractile function at follow-up. The flow pattern characteristic of poor tissue perfusion shows rapid diastolic deceleration together with a poor and even negative systolic component. This pathological pattern of coronary flow velocity is due to increased microvascular resistance and reduced intramyocardial blood volume arising from the severe ischemic damage.9 Microvascular impairment impedes the normal emptying of blood during systole toward the venous system and, under the worst conditions, the flow returns retrogressively toward the epicardial vessel (negative protosystolic component). On the other hand, the low blood volume in the critically ischemic myocardium leads to fast filling in the diastolic phase, thus explaining the increase in diastolic deceleration.
In our study, revascularization was not done in the acute phase of the infarction but during the first month. However, our results continue to confirm the presence of a different flow pattern between patients with viable myocardium (shown by a smaller akinetic area together with greater recovery of regional contractility at follow-up) and those with more severe and persistent changes in contractility at follow-up. The diastolic deceleration halftime was the only phasic coronary flow parameter which differed in both groups, both at baseline and under hyperemia: patients with viable myocardium presented a significantly greater diastolic deceleration halftime in the culprit artery, indicating better myocardial filling during the diastolic phase.
Coronary Flow Reserve in the Culprit Artery
The CFR can be initially impaired after percutaneous revascularization, even though the angiographic result is good.10 This phenomenon is especially evident in AMI.11 In this context, several mechanisms can account for a pathological coronary reserve after eliminating the epicardial obstruction: hyperemic flow at baseline, reversible increased resistance in the microcirculation or microvascular stunning, or the passive increase in microvascular resistance by tissue destruction and distal embolization of the components of an occluding thrombus.
In our study there were no significant differences in CFR among patients with and without viable myocardium in the post-PCI study. However, an interesting finding is the increase in CFR occurring at follow-up only in the group of patients with viable myocardium, which is basically due to increased flow velocity under hyperemia. This evolution indicates the presence of an initial reversible microvascular resistance in patients with a greater amount of viable myocardium.
Although the CFR did not undergo changes between initial control and follow-up in the RC group as a whole, when analyzed individually there was an increase in coronary reserve in half of the cases (4 patients). The small study population places limits on what can be concluded and it is possible that factors other than viability are involved in the recovery of CFR. In any case nevertheless, the CFR seems to undergo a different evolution in the culprit artery depending on viability in its myocardial territory, which represents a new finding.
Convergence of Coronary Flow Characteristics in the Culprit Artery at Follow-up, Independently of Preserved Myocardial Contractility
Some 4-6 months after revascularization, coronary flow in the culprit artery is uniform with no difference between patients with virtually preserved regional contractile function and those with a great area of dyskinetic myocardium. This convergence holds for both the CFR and the phasic coronary flow characteristics, and specifically for diastolic deceleration halftime.
Limitations
The main limitation of this study is the small number of patients, although it is similar to that of other studies with similar aims. Given that the coronary flow patterns were studied following PCI, the conclusions drawn cannot be used to make decisions regarding the revascularization of a lesion as a function of the viability of its territory. On the other hand, carrying out PCI within the first month of the infarction can present a limitation, since it covers a sufficiently broad period of time such that the coronary flow characteristics could undergo spontaneous variations. Another limitation is the different location of the infarction in the groups with and without myocardial viability and, thus, the possibility of different arteries being involved; taking into account that the flow pattern can present differences depending on the coronary artery, it cannot be ruled out that the differences between viable and non-viable flow patterns could influence the specific characteristics of each artery analyzed. Furthermore, due to the small sample size, it is not possible to calculate a cut-off value for the deceleration halftime that could be a predictor of viability. Finally, the conclusions obtained do not necessarily apply to certain groups of patients (patients with diabetes, multivessel disease, severe ventricular dysfunction, etc) that were barely represented in the study or otherwise excluded.
CONCLUSIONS
In patients with recent myocardial infarction, coronary flow in the culprit artery shows a different pattern according to the extent of viable myocardium. In the early analysis following revascularization, the diastolic deceleration halftime was the only phasic coronary flow characteristic that differentiated viable and non-viable vascular territories. Some 4-6 months after revascularization, the differences in the coronary flow characteristics between patients with and without myocardial viability disappeared. The increase in coronary flow reserve at follow-up in patients with a greater area of viable myocardium could indicate the initial presence of reversible microvascular resistance.
ABBREVIATIONS
PCI: percutaneous coronary intervention.
CFR: coronary flow reserve.
FFR: fractional flow reserve.
Correspondence: Dr. R.J. Ruiz-Salmerón.
Departamento de Hemodinámica y Cardiología Intervencionista. Hospital Clínic.
Villarroel, 170. 08036 Barcelona. España.
E-mail: rjruiz@clinic.ub.es