The new coronavirus SARS-CoV-2, which gives rise to the highly contagious COVID-19 disease, has caused a pandemic that is overwhelming health care systems worldwide. Affected patients have been reported to have a heightened inflammatory state that increases their thrombotic risk. However, there is very scarce information on the management of thrombotic risk, coagulation disorders, and anticoagulant therapy. In addition, the situation has also greatly influenced usual care in patients not infected with COVID-19. This article by the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology aims to summarize the available information and to provide a practical approach to the management of antithrombotic therapy.
Keywords
The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which gives rise to the highly contagious COVID-19 disease, has caused a worldwide pandemic. The disease has a wide clinical spectrum ranging from patients who are asymptomatic or with mild respiratory symptoms to those with severe viral pneumonia, respiratory failure, dyspnea, multiple organ failure, and death.1 Affected patients have been reported to have a heightened inflammatory state that increases their thrombotic risk. Drugs used in the treatment of viral infection and its complications interact with other drugs, in particular with antithrombotic drugs, hampering their prescription and raising questions regarding daily clinical practice. However, there is little information on how to address thrombotic risk, coagulopathy, and anticoagulant therapy in these patients.
We emphasize that this disease is highly contagious and is overwhelming health care systems worldwide. Recently, there has been a significant reduction in health care activity during the COVID-19 epidemic in Spain and in particular a marked decrease in the number of patients treated with primary angioplasty.2 There will likely be a negative prognostic impact with an increased risk of morbidity and mortality because of delayed requests for medical attention, and difficulties in transfers and in the provision of care by overwhelmed hospitals. The disease is also affecting primary care services and specialist outpatient visits. Thus, even patients who are not infected with SARS-CoV-2 are being affected by the pandemic, which is having a strong influence on the optimization of antithrombotic therapy due to the current health care situation.
This article was prepared by the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Its aim is to summarize the available information and provide simple guidelines for the use of antithrombotic drugs in order to guarantee optimal care for patients infected by the SARS-CoV-2 virus. It also addresses the use of these drugs in uninfected patients, whose care may be influenced by the current situation.
MECHANISMS PARTICIPATING IN THE PROTHROMBOTIC STATE OF SARS-CoV-2 INFECTIONMost patients affected by SARS-CoV-2 experience a flu-like illness with mild symptoms such as fever, cough, and some degree of dyspnea. However, in a smallpercentage of patients, a pneumonia-like condition develops that can give rise to acute respiratory distress syndrome, septic shock, metabolic acidosis, and coagulopathy that can lead to a condition which shares some of the characteristics of disseminated intravascular coagulation (DIC) and multiple organ failure.3
In patients with septic shock, the development of coagulopathy typically implies a worse prognosis, which has been corroborated by several publications on series of patients affected by COVID-19. Thus, these patients have higher D-dimer levels, which is associated with a worse prognosis and even mortality.4,5 Therefore, levels that are 2 to 3 times more than the normal value should be taken into account, even in the presence of mild symptoms.6,7 In addition, a slight increase in prothrombin time has been observed in patients with severe symptoms. By contrast, thrombocytopenia, which is considered an indicator of mortality from sepsis, is not usually observed in these patients, although its presence is a clear indicator of poor prognosis and is associated with a 5-fold enhanced risk of severe COVID-19.8 Tang et al.5 found that 71% of nonsurvivors met the International Society of Thrombosis and Haemostasis (ISTH) diagnostic criteria for DIC.
The pathophysiology of coagulopathy is complex due to the interrelationship between the cellular and plasma elements of the hemostatic system and the components of the innate immune response. The host responds to infection by activating the cellular components of the innate immune system, inducing the production of cytokines along with the expression of tissue factor.9 Increased cytokine levels may be the cause of lung inflammation and impaired gas exchange, which in turn would accelerate pulmonary fibrinolysis and lead to increased D-dimer levels.10 Furthermore, increased tissue factor expression is a relevant activator of the hemostatic system. Finally, activation of the endothelium, platelets, and other leukocyte components will also lead to an imbalance in thrombin production giving rise to consequent fibrin deposition with subsequent microangiopathy and tissue damage.11
Furthermore, it is reasonable to consider that patients hospitalized for COVID-19 are at increased risk of venous thromboembolic disease (VTE), particularly those admitted to an intensive care unit. These patients have reduced venous flow due to prolonged bed rest, prothrombotic changes, and endothelial damage (ie, the 3 elements of the Virchow triad), which may be secondary to binding of the virus to angiotensin-converting enzyme II receptor.12 Therefore, these patients are candidate for thromboprophylaxis with low-molecular-weight heparin (LMWH) or mechanical devices, depending on the risk of bleeding.
The ISTH recommends that D-dimer, prothrombin time, platelet count, and fibrinogen should be determined and monitored to stratify patients and identify those with poor prognosis for further intensive monitoring and even treatment modification. It should be noted that bleeding events are relatively common in these patients and, if they do occur, treatment should aim to substitute and maintain the platelet count at more than 50 × 109/L, fibrinogen more than 2.0g/L, and prothrombin time ratio less than 1.5.7
ANTITHROMBOTIC DRUG AND COVID-19 DRUG INTERACTIONSAlthough there are no specific treatments for SARS-CoV-2, a number of drugs are under investigation and have been made available for compassionate use in clinical practice.13Table 1 shows interactions between antithrombotic drugs and the main drugs used to treat SARS-CoV-2.
Antiretroviral drugs such as lopinavir/ritonavir are potent cytochrome P450 3A4 (CYP3A4) inhibitors and may therefore increase the concentrations of direct-acting oral anticoagulants (DOAC), mainly rivaroxaban and apixaban, as well as concentrations of ticagrelor, thus discouraging their use.14 Caution should be exercised in their concomitant use with dabigatran, edoxaban, and vitamin K antagonists (VKA). Some studies have found decreased concentrations of the active metabolites of clopidogrel in patients receiving concomitant antiretroviral therapy.15 The antiretroviral drug combination darunavir/cobicistat, which is another potent CYP3A4 inhibitor, is also a P-glycoprotein inhibitor and therefore its combination with any of the DOACs or with ticagrelor should be avoided. The use of intravenous antiplatelet agents is of little concern because they are metabolized independently of liver function. Preliminary data on remdesivir and immunomodulatory drugs show no significant interactions.
Chloroquine and hydroxychloroquine are moderate CYP2D6 and P-glycoprotein inhibitors. They have little interaction with apixaban and rivaroxaban, but caution should be exercised when administered with dabigatran and edoxaban.16 Finally, methylprednisolone can interact with VKAs, and so their concomitant prescription is discouraged.
ANTICOAGULANT THERAPY FOR PATIENTS ADMITTED WITH COVID-19Patients with SARS-CoV-2 infection are at increased risk of thromboembolic events, especially VTE, which is associated with the critical situation and immobilization entailed by this disease. These critically ill patients are at increased thromboembolic risk, making effective VTE prevention strategies crucial.17 Studies conducted in the Wuhan population found a high incidence of VTE (up to 20% of patients admitted to intensive care units) associated with high mortality.18,19 However, optimal prophylactic and therapeutic anticoagulation strategies during hospitalization are not clearly established.20
Thachil et al.7 suggested that prophylactic doses of LMWH should be considered for all patients needing hospitalization in the absence of contraindications (such as active bleeding or platelet count less than 25 ×109/L) with dose adjustment in patients with marked D-dimer elevation and those with severe symptoms. Studies related to SARS found that initial treatment with LMWH reduced the 7-day mortality rate by 48% and the 28-day mortality rate by 37%. It also significantly improved the partial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by mitigating the formation of microthrombi and associated pulmonary coagulopathy.21 Furthermore, a study of critically ill patients found that the use of LMWH led to a decrease in inflammation.22 Therefore, the studies related to COVID-19 used prophylactic doses (enoxaparin 40-60mg/d) of LMWH during hospitalization in all patients for at least 7 days. The use of LMWH reduces thrombin generation and venous thromboembolic events (ie, deep vein thrombosis or pulmonary thromboembolism). Furthermore, LMWH is known to have anti-inflammatory properties, which can help to control this disease in which there is a marked increase in proinflammatory cytokines. Based on the 2-way relationship between inflammation and thrombosis or “immunothrombosis”, thrombin blockade by LMWH can buffer the inflammatory response23 and reduce endothelial damage.24 In a recent study with 449 patients, Tang et al.25 found that the use of LMWH led to a reduction in mortality in patients who met the criteria for DIC or with D-dimer more than 6-fold the upper limit of normality. Liny et al.26 found that patients with D-dimer more than 4-fold the upper limit of normality who met the criteria for DIC had distal ischemic abnormalities; they recommended a regimen of LMWH 100 IU/kg/12h for 5 days.
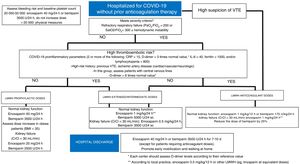
An algorithm for the treatment approach to these patients is shown in figure 1. The first step of the algorithm is to prescribe LMWH to all patients requiring hospitalization, with weight-adjusted doses for patients with a body mass index of more than 35 after assessing bleeding risk and baseline platelet count. The patient's thromboembolic risk and severity of COVID-19 should be assessed, according to which intermediate/extended or therapeutic LMWH dose will be prescribed; however, there is a lack of evidence in this regard, because intermediate doses were used in the case series showing a reduction in mortality with LMWH.24 Proinflammatory and hemostatic parameters should be monitored every 24 to 48hours (depending on clinical severity), according to which the patient's risk, and therefore the LMWH dose, will be reassessed. Stable patients may be discharged and convalesce at home. This period of immobilization may be prolonged and lead to an increase in thromboembolic events and mortality. Thus, it is recommended to prolong the use of LMWH in prophylactic doses for 7 to 10 days postdischarge. If a diagnosis of VTE is established, LMWH should be administered at therapeutic doses; it is could be relevant to determine anti-Xa activity 48hours after the start of anticoagulation therapy to ensure efficacy and minimize the risk of bleeding.
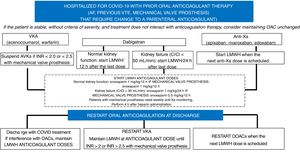
Patients with indications for anticoagulation therapy prior to SARS-CoV-2 infection usually have atrial fibrillation or VTE and have mechanical valve prostheses. Figure 2 shows an algorithm for the treatment approach to patients with prior oral anticoagulation therapy admitted for COVID-19 infection, in which a change to parenteral anticoagulation is proposed (mainly due to the severe situation or to interactions with COVID-19 drugs). There is no clear evidence regarding the maintenance of oral anticoagulation therapy in patients admitted for SARS-CoV-2 infection, although a priori it could be maintained in patients who are stable and not taking any drug that could potentially cause an interaction. Therefore, potential drug-drug interactions and the severity of the patient must be carefully assessed to apply the correct algorithm to switch from oral anticoagulation therapy to anticoagulant doses of LMWH and thus minimize the potential thromboembolic and bleeding events of incorrect bridge therapy. This approach should be followed again when antiviral therapy ends and oral anticoagulation can be r-administered.
Antithrombotic approach to patients admitted for covid-19 with prior anticoagulant therapy. AF, atrial fibrillation; CrCl, creatinine clearance; DOAC, direct-acting oral anticoagulant; INR, international normalized ratio; LMWH, low-molecular-weight heparin; OAC, oral anticoagulation; VKA, vitamin K antagonists; VTE, venous thromboembolic disease.
During the current SARS-CoV-2 infection pandemic, there has been a clear reduction in the invasive approach to patients with acute coronary syndrome. Scientific societies have recommended delaying nonurgent procedures to avoid infections and prevent the collapse of the health system.27 Furthermore, although primary angioplasty is the preferred reperfusion strategy in patients with ST-segment elevation acute myocardial infarction, fibrinolysis can be considered in patients with an estimated time from diagnosis to coronary intervention of more than 120minutes, in infected patients with poor clinical status that hinders transfer, or in those at low risk of bleeding and with symptom onset less than 3hours previously.27 Despite these considerations, there are clearly a significant number of patients, with or without SARS-CoV-2 infection, who are admitted after an acute coronary syndrome and undergo coronary intervention.
In uninfected patients, the indications for antiplatelet therapy should continue to be those recommended in the current clinical practice guidelines.28,29 However, in patients with SARS-CoV-2 infection, there are 2 factors that may lead to modifications to the antiplatelet therapy strategy. One factor is the high inflammatory and prothrombotic component that this infection appears to have, and the other is potential drug-drug interactions between COVID-19 drugs and antiplatelet agents.
As discussed in the previous section, there have been reports of interactions (related to CYP3A4) between some of the antiviral drugs used, particularly lopinavir/ritonavir and darunavir/cobicistat, and clopidogrel and ticagrelor. These interactions decrease the formation of the active metabolite of clopidogrel and thus decrease its antiplatelet efficacy, whereas they increase the concentrations of ticagrelor and thus increase its antiplatelet efficacy. In fact, these drug combinations are contraindicated. For this reason, the use of prasugrel has been proposed in these patients, although with qualifications. Currently, the efficacy of these antiviral drugs remains controversial.30 Thus, treatment should be individualized if the standard antiplatelet strategy needs to be changed. For example, there may be an increased risk of bleeding in patients who would normally receive clopidogrel (due to the balance between ischemic and hemorrhagic risk), but who are switched to prasugrel as standard treatment. If any of the antiviral drugs that interact with clopidogrel or ticagrelor have to be used, then prasugrel should be prescribed during the period these antivirals are administered. Nevertheless, it is important to take into account contraindications (eg, previous stroke) and other factors (eg, age > 75 years, weight <60kg, history of bleeding). The suspension of these antivirals depends on local protocols, but they are usually prescribed for a few days or for no more than 1 to 2 weeks. Once antivirals are stopped, thus eliminating the risk of drug interaction, the prescribed P2Y12 inhibitor should be changed to the P2Y12 inhibitor that would have been selected under normal circumstances.31 If this change is performed in the acute phase of an event, loading doses of the drug must be administered.28
Acute coronary syndromes with large thrombotic loads can occur in the context of an infection with high inflammatory and prothrombotic potential. In such cases, powerful parenteral antiplatelet agents such as glycoprotein IIb/IIIa inhibitors or cangrelor can be considered for use. Cangrelor is of particular interest if there has been inadequate pretreatment with oral P2Y12 receptor inhibitors, although the risk-benefit ratio should always be taken into account. There are no pharmacological interactions between these parenteral antiplatelet agents and COVID-19 drugs.
ANTITHROMBOTIC THERAPY FOR PATIENTS WITHOUT COVID-19 INFECTIONIndications for the use of antiplatelet therapy in patients without COVID-19 infection have not been changed and should not be changed. The indications remain the same as they were before the pandemic, but the way in which we communicate to our patients has changed, and indications and changes to treatment might be assessed by means other than the physical presence of the patients. A distinction must be made between new indications for antiplatelet therapy and treatment extensions. The current pandemic has led to the cancellation of most elective procedures and to the recommendation of conservative treatment for chronic patients.
For patients diagnosed with stable angina not requiring invasive treatment, aspirin is the antithrombotic drug of choice. For patients referred for percutaneous revascularization, dual antiplatelet therapy with aspirin and clopidogrel is indicated. However, acute coronary syndrome should be treated by cardiac catheterization and early discharge to reduce potential contagion in settings with a high prevalence of COVID-19 infection, such as hospitals.27 There should be no change in antiplatelet therapy in patients without COVID-19 infection. Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor is indicated and prasugrel and ticagrelor should be prioritized over clopidogrel.
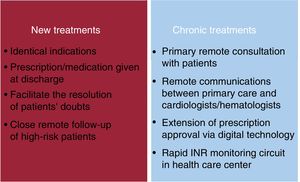
Measures must be implemented to ensure that patients have prescribed drugs available at all times (figure 3). It should also be borne in mind that the pandemic can lead to delays in the approval of prescribed drugs, problems going to pharmacies, overwhelmed pharmacies, and a lack of supplies. All this is happening at a time when patients have been told not to visit hospitals or health care centers except for urgent reasons. For stable patients without COVID-19 infection, any change of antiplatelet therapy should be guided by ischemic and bleeding risks. Therefore, actions should be encouraged to avoid the physical presence of patients in any type of health care facility to reduce the risk of infection.
Unless patients are admitted for an invasive or surgical procedure, there is no reason to stop oral anticoagulant therapy. Suspension for these reasons must be performed following the recommendations of the Spanish Society of Cardiology consensus document on antithrombotic therapy and invasive procedures.32
After discharge, anticoagulated patients should continue with their usual therapy. If anticoagulant therapy has been withdrawn during hospitalization, instructions for its reintroduction should be accurately stated in the discharge report. During the lockdown, the government should extend the approval of DOAC prescriptions and encourage remote communication between family physicians and hematologists and cardiologists to resolve issues, adjust doses, or reintroduce therapy in the event of a surgical procedure.
In the case of VKA therapy, the situation is more complex. During hospitalization, if there are no surgical or invasive procedures or significant drug interactions, patients should continue with this treatment without bridge therapy and with international normalized ratio (INR) monitoring every 4 to 5 days, as needed. During their hospital stay, many patients taking VKAs tend to experience coagulation imbalances, which require close INR monitoring at discharge. INR monitoring at 7 to 8 weeks should only be considered for patients with good therapeutic control. To minimize the need for monitoring in the current situation, if patients are eligible for DOAC prescriptions, changes during hospitalization should be prioritized, thus avoiding visits to health care centers for subsequent monitoring after discharge. The health departments of some autonomous communities (eg, Andalusia, the Community of Madrid, the Region of Murcia, the Valencian Community) have approved anticoagulation therapy with DOACs for patients with a recent diagnosis of atrial fibrillation to avoid the frequent monitoring required at the start of VKA therapy. The Spanish government should increase the indications for which DOACs are funded by the public health care system and extend the time approved prescriptions are validated. There should be close communication between primary care physicians and hematologists/cardiologists.
When VTE is the reason for hospitalization, patients should continue with LMWH until the end of the lockdown period. When patients are receiving anticoagulant therapy for VTE prior to hospitalization and have good therapeutic control, INR monitoring should be done just prior to discharge to delay the next monitoring visit for as long as possible. When there is poor therapeutic control, patients should receive LMWH. It should be noted that the Spanish National Health System does not fund DOACs for the treatment of VTE.
In the current situation, special attention should be paid to patients with mechanical valve prostheses as these patients are especially vulnerable. Switching to LMWH should only be done for short periods.33 The health care center should establish a specific route for these patients, so that INR monitoring can be conducted as quickly as possible and patients can collect their treatment prescription during the same visit. Alternatively, patients could receive their anticoagulant therapy prescription via digital means. If VKAs are switched for LMWH, it should be noted that this is an off-label indication and that LMWH should be administered every 12hours with anti-Xa monitoring.
CONCLUSIONS AND LIMITATIONS OF THIS DOCUMENTUnfortunately, there is no solid scientific evidence to support antithrombotic therapy in patients infected with COVID-19. We are working in an ever-changing scenario in which the benefit of specific antiviral therapy has not even been clearly demonstrated. Most of the recommendations provided in this consensus document are based on the opinion of the authors or on very small series of patients. In addition, almost all the information available is based on series of patients from China. From what is being seen, the pandemic behaves in the western world in quite a different way to that reported in China. For all these reasons, the patients’ clinical situation and the presence of comorbidities should always be taken into account to assess both thrombotic and bleeding risk.
Finally, now that the virulence of the pandemic seems to be decreasing, we invite readers to reflect on how we have acted in this first phase of the pandemic, because we must identify our successes and errors so that we can be prepared for a possible second wave, which has been predicted by many experts. Currently, the highest priorities are to correctly manage infected patients to avoid fatal events and to ensure the health of the uninfected population.
CONFLICTS OF INTERESTD. Vivas has received conference attendance expenses from Eli Lilly & Co., Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Boehringer Ingelheim, and Bristol-Myers-Squibb, and has undertaken consultancy for AstraZeneca, Eli Lilly & Co., Bayer, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Bristol-Myers-Squibb. V. Roldán has received conference attendance expenses from Pfizer-BMS, Daiichi Sankyo, and Boehringer Ingelheim, and has undertaken consultancy for AstraZeneca, Daiichi Sankyo, and Boehringer Ingelheim. I. Roldán has received conference attendance expenses from Eli Lilly, Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Boehringer Ingelheim, and Bristol-Myers-Squibb, and has undertaken consultancy for AstraZeneca, Eli Lilly & Co., Bayer, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Bristol-Myers-Squibb. A. Tello-Montoliú has received fees for consultancy and a research project from AstraZeneca. J.M. Ruiz-Nodar has received conference attendance expenses from AstraZeneca, Biosensor, Boston Scientific, Medtronic, and Terumo. J. Cosín-Sales has received conference attendance expenses from Daiichi Sankyo, Bayer, Pfizer-BMS, and Boehringer Ingelheim. L. Consuegra has received conference attendance expenses from Daiichi Sankyo, Abbott, Esteve, and AstraZeneca. J. Luis Ferreiro has received conference attendance expenses from Eli Lilly & Co., Daiichi Sankyo, AstraZeneca, Roche Diagnostics, Pfizer, Abbott, Boehringer Ingelheim, and Bristol-Myers-Squibb, has undertaken consultancy for Astra Zeneca, Eli Lilly & Co., Ferrer, Boston Scientific, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Bristol-Myers-Squibb, and has received research grants from AstraZeneca. F. Marín has received conference attendance expenses from Daiichi Sankyo, AstraZeneca, and Pfizer-BMS, has undertaken consultancy for Astra Zeneca, Daiichi Sankyo, and Boehringer Ingelheim, and has received research grants from AstraZeneca. All the other authors declare no conflicts of interest.