In recent years, coronary computed tomography angiography has become an increasingly safe and noninvasive modality for the evaluation of the anatomical structure of the coronary artery tree with diagnostic benefits especially in patients with a low-to-intermediate pretest probability of disease. Currently, increasing evidence from large randomized diagnostic trials is accumulating on the diagnostic impact of computed tomography angiography for the management of patients with acute and stable chest pain syndrome. At the same time, technical advances have substantially reduced adverse effects and limiting factors, such as radiation exposure, the amount of iodinated contrast agent, and scanning time, rendering the technique appropriate for broader clinical applications. In this work, we review the latest developments in computed tomography technology and describe the scientific evidence on the use of cardiac computed tomography angiography to evaluate patients with acute and stable chest pain syndrome.
Keywords
In 2010, 1 in of every 6 deaths in the United States of America was related to coronary heart disease with estimated direct and indirect costs of ∼$204.4 billion.1 With 6.9 million patients out of 136.3 million emergency department (ED) visits in 2011, chest pain was one of the 20 leading primary diagnostic groups.2 However, only 17% of patients admitted to the ED met the criteria for acute coronary syndrome (ACS), while 55% showed noncardiac causes.3 When ACS is suspected, the evaluation should include medical history, physical examination, electrocardiogram (ECG), and cardiac injury markers, such as troponin. Patients with a very low probability of myocardial infarction (MI) (< 5%) can be well identified and hence be admitted to an observation unit for further risk stratification examinations, such as exercise treadmill, cardiac single photon emission computed tomography (SPECT), cardiac magnetic resonance tomography, or stress echocardiography.4,5 This approach may eventually result in increased admission rates and unnecessary noninvasive or invasive follow-up tests, which will ultimately dramatically increase costs.6
In the last decade, coronary computed tomography angiography (CCTA) has been established as a safe alternative modality for the evaluation of coronary artery disease (CAD), especially in patients with a low or intermediate pretest probability for coronary obstruction, which was duly endorsed by the American College of Cardiology/American Heart Association.7 Recently, randomized trials, which compared the use of CCTA with the current standard of care (SOC), has provided increasing evidence that the application of CCTA in routine practice can safely reduce hospital stay and hospital costs.8
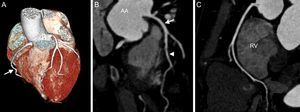
RECENT TECHNICAL DEVELOPMENTWith the introduction of multidetector technology in 1999, visualization of the coronary artery tree at low heart rates became feasible.9 Since then, computed tomography (CT) technology has evolved rapidly, including an increasing number of detectors of up to 392 rows, the introduction of dual-source-CT technology, or increasing pitch factors, enabling image data acquisition in a single heartbeat.10 The increasing spatial resolution of up to approximately 0.5mm allows assessment of the coronary arteries, as well as the presence of plaque and stenosis. Major currently-available technical improvements include imaging at low voltage and high-pitch factors, as well as the introduction of iterative reconstruction (IR) algorithms (Figure 1).
Cardiac computed tomography angiography of a 47-year-old man presenting with atypical chest pain syndrome and known hyperlipidemia. Prospective, elecrocardiogram-triggered computed tomography angiography (100kV, 2.1 mSv) demonstrates a right dominant coronary artery system on volume-rendered images (A, arrow). B: Curved multiplanar reconstruction shows no evidence of coronary plaque or stenosis in the left main coronary artery (arrow), or left anterior descending coronary artery (arrowhead). Similarly, no coronary plaque or stenosis is detected in the right coronary artery (C). AA: ascending aorta; RV: right ventricle.
Lowering the tube voltage has been introduced as a means to reduce radiation exposure in patients with lean body habitus.11 However, at the same time, a reduction of tube voltage is associated with an increase in image noise, which may therefore impair diagnostic accuracy and could require increased tube current, which is available in more recent generations of scanners.12 It is also known that the attenuation value of iodinated enhancement is increased in a lower tube voltage, and hence the administration of contrast media can be reduced, which particularly benefits patients with renal impairment.13 Early multicenter and multivendor studies, such as the Protection I trial, showed a 53% reduction in radiation dose estimates with no significant impairment of diagnostic image quality when the tube voltage was lowered from 120kV to 100kV.14 A recent study comparing CCTA with 3 tube voltage settings showed a significant reduction in radiation dose when comparing 70kV with 80kV and 100kV (0.44 vs 0.78 and 0.92 mSv; P<.0001). The reduction in tube voltage was associated with a significant increase in noise in the lowest kV-setting (P<.0497) but with no apparent impairment in subjective and qualitative image quality. The latest study with 43 patients undergoing a CCTA examination with a tube voltage setting at 70kV prior to the planned invasive angiography, showed very high diagnostic accuracy (sensitivity of 92.2% and specificity of 89.5%) while reducing the dose estimate to 0.2 mSv.15
HIGH-PITCH ACQUISITIONSAnother strategy to reduce the dose estimate is to increase the pitch,16 which is defined as the table travel per rotation divided by the nominal slice.17 In a single-source CT system, the pitch was limited to 1.5, due to data loss at higher pitch values. With the introduction of the dual-source-CT technology system, the pitch could be increased to over 3, as the second source/detector separately acquires the data one quarter rotation later without a gap.18 Hence the radiation exposure can be lowered significantly using the technology, as no slice overlap is needed.17 In a phantom and patient study, Sommer et al19 compared the high-pitch protocol set at 3.4 with the conventional prospective triggered acquisition and the retrospective ECG gated acquisitions (pitch=0.2). The phantom based radiation dose estimate showed the lowest value in the high-pitch protocol compared with the prospectively ECG-triggered and retrospective ECG-gated acquisition (1.21 vs 3.12 vs 11.81 mSv). In the patient substudy, the radiation dose estimate showed a similar trend (1.11 vs 4.15 vs 11 mSv; P<.001) and significant differences in motion-free display of coronary arteries (99% vs 87% vs 92% for the high-pitch, prospective ECG-triggered and retrospective ECG-gated acquisition, respectively). In a similar study with 50 patients, the application of the high-pitch protocol with a tube voltage at 100kV resulted in an estimated radiation dose of 1 mSv in nonobese patients with low and stable heart rate.20
ITERATIVE RECONSTRUCTION TECHNIQUESThe introduction of IR techniques have had a major impact on reducing radiation exposure. Until recently, conventional CT image reconstruction from raw data attenuation measurement was based on the filtered back projection (FBP) technique.21 Images based on FBP take into account multiple projections from different scanning angles by back-projecting raw data; however, this does not consider either the statistical noise or the X-ray beam geometry or the photon interaction with the scanned object and detector.22 When a standard radiation dose is applied, FBP is well accepted; however, when photon density and hence radiation dose is reduced, there is an incremental increase in image noise. To overcome this problem, IR can be applied to enhance the image quality. Iterative reconstruction is based on a mathematical model, iterating the image reconstructions several times and hence generating images with lower noise.22–24 In one study, 60 patients were referred for invasive angiography and 2 CCTA examinations: one CCTA-acquisition with conventional acquisition settings and FBP reconstruction technique and a second CCTA-acquisition with a reduction of the tube current-time product by 50% using the IR technique, showing no significant difference in diagnostic accuracy and image quality between the FBP and IR technique.25 In a very low-dose (100kV) study, prospectively ECG-gated CCTA images were reconstructed from the raw data using the FBP and IR techniques. The IR technique significantly improved image quality and diagnostic accuracy (sensitivity: 81% vs 69%; specificity: 97% vs 97).26
SCIENTIFIC EVIDENCE IN THE SETTING OF ACUTE CHEST PAINAcute chest pain is often a leading symptom of ACS, pulmonary embolism, aortic dissection, or even esophageal pathology. Most persons admitted to the ED with acute chest pain are routinely evaluated for ACS. Acute coronary syndrome is defined as decreased blood flow in the coronary arteries due to narrowing of blood vessels, at worst due to occlusion of the coronary artery leading to ischemia of the myocardial muscle.27 Spasm, calcified and noncalcified plaques, as well as thrombosis can result in a decrease in coronary artery lumen diameter and mismatch between oxygen supply and demand.27 Coronary computed tomography angiography, using intravenous administration of iodinated contrast media, has been largely accepted as a noninvasive modality to visualize the coronary arteries, primarily for its excellent negative predictive value in ruling out CAD.
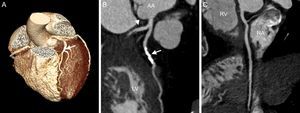
Noncontrast coronary artery CT, such as coronary artery calcium scan, can be performed rapidly (3-5 s breath hold) with a radiation exposure of up to 0.37 mSv, when newer protocols, such as high-pitch scanning and IR, are used.28,29 The advantage of coronary artery calcium scan consists in its highly standardized interpretation (Agatson score determined by the sum of the weighted scores for all coronary arteries multiplied by the maximal lesion density).30 The patient's score is then compared with similar results obtained in asymptomatic persons of the same sex, age, and ethnicity.31 A score above the 75th percentile is considered “high-risk”.32 In symptomatic patients, a negative calcium score is often unreliable in excluding significant CAD, since the coronary artery calcium scan is unable to visualize noncalcified plaques, which may attain marked obstructive severity in 1% to 3% of patients.33,34 Therefore, for most practitioners, CCTA remains the diagnostic modality of choice in evaluating ACS (Figure 2).
Cardiac computed tomography of a 72-year-old female patient presenting with acute chest pain to the emergency department. A: Prospective, elecrocardiogram-triggered computed tomography angiography (100kV, 1.8 mSv). B: Curved multiplanar reconstruction shows a significant luminal narrowing due to a calcified plaque in the left anterior descending artery. C: No coronary plaque or stenosis in the proximal circumflex (arrowhead). No coronary plaque or stenosis is detected in the right coronary artery. AA, ascending aorta; LV, left ventricle; RA, right atrium; RV, right ventricle.
To date, 4 randomized controlled trials have been published (Table).35–38 In a study with 197 patients, Goldstein et al35 aimed to compare the safety, diagnostic efficacy, and efficiency of CCTA compared with the SOC, which included cardiac biomarkers and same-day rest-stress myocardial perfusion SPECT. Outcomes were defined as freedom from major adverse events over a period of 6 months, diagnostic efficacy, cost, and length of care. Coronary computed tomography angiography-track was able to exclude or identify CAD as the source of chest pain in 75% of patients, reduced the time to diagnosis (3.4h vs 15h; P<.001), and was lower in cost ($1586 vs $1872; P<.001).
Overview of Randomized Controlled Trials Comparing Coronary Computed Tomography Angiography With Standard of Care for the Evaluation of Acute Chest Pain in an Emergency Department Setting
| Study | Patients, No. | Study design | Follow-up (months) | MI in follow-up | Time (CT vs SOC) | Cost (CT vs SOC) |
|---|---|---|---|---|---|---|
| Goldstein et al35 | 197 | CCTA vs SPECT | 6 | 0 | 3.4 h vs 15 h; P<.001 | $1586 vs $1872; P<.001 |
| CT-STAT36 | 669 | CCTA vs SPECT | 6 | 0 | 2.9 h vs 6.3 h; P<.0001 | $2900 vs $4297; P<.0001 |
| AC RIN-PA37 | 1370 | CCTA vs SOC | 1 | 0 in the negative CCTA-arm | 18h vs 24.8 h; P<.001 | Not reported |
| ROMICAT II38 | 1000 | CCTA vs SOC | 1 | 0 | 23.2h vs 30.8 h; P<.001 | $4289 vs $4060, P=.65* |
CCTA, coronary computed tomography angiography; CT, computed tomography; MI, myocardial infarction; SOC, standard of care; SPECT, single photon emission computed tomography.
Patients who underwent a CCTA workup required fewer repeat evaluations for recurrent chest pain compared with patients who were referred to the SOC arm (2% vs 7%; P=.10).
In the multicenter randomized CT-STAT Trial conducted in 16 ED, 669 patients suspected of having CAD were allocated to either CCTA or SPECT.36 Outcomes were defined as time to diagnosis, major adverse events, costs, and safety. Compared with SPECT, CCTA showed no difference in major adverse events but was able to reduce the time to diagnosis by 54% (median 2.9h vs 6.3h; P<.0001) and costs by 38% (median $ 2900 vs 4297; P<.0001).
In The American College of Radiology Imaging Network of Pennsylvania trial (AC RIN-PA), 1370 low-to-intermediate risk patients were randomly assigned in 5 centers to CCTA or SOC in a 2:1 ratio.37 The study aim was to evaluate the frequency of MI and cardiac deaths in the negative CCTA group in the first 30 days following the examination. Among the 640 patients with a negative CCTA examination, there were no cardiac deaths and no MI. Patients in the CCTA group had a higher rate of discharge from the ED and a shorter hospital stay compared with those in the SOC group (49.6% vs 22.7%, 18h vs 24.8h; P<.001 respectively). Additionally, CCTA had a higher rate of CAD-detection (9.0% vs 3.5%).
In the ROMICAT II trial, 1000 patients with symptoms suggestive ACS were randomly assigned to either CCTA or SOC in a 1:1 ratio.38 Patients who underwent CCTA showed a 7.6-hour reduction in mean stay and were more frequently discharged directly from the ED compared with those in the SOC group (47% vs 12%; P<.001). There were no significant major adverse events 28 days after presentation to the ED. Due to more downstream testing, the cumulative mean costs of care in the CCTA-arm were similar to those of the SOC-strategy ($4289 vs $4060, P=.65). Additionally, the CCTA-strategy was associated with higher radiation exposure.
In a meta-analysis by Hulten et al,8 summarizing the data from the above trials, there were no cardiac deaths after discharge and there was no difference regarding the incidence of MI or reassessment between patients who underwent CCTA and SOC. Although CCTA in evaluating the acute chest pain in ED was associated with a decrease in the length of stay and cost savings, patients in the CCTA arm were more likely to undergo an invasive coronary angiography (7.5% vs 5.6%; P=.03) or even a revascularization procedure (4.2% vs 2.2%; P=.004) but without a defined different outcome.
A recent prospective study by Mas-Stachurska et al39 compared the diagnostic performance of CCTA and exercise echocardiography in patients with acute chest pain, normal ECG, negative troponin markers, and a low-to-intermediate probability of CAD. Acute coronary syndrome was confirmed in 17 out of 69 patients (24.6%) by invasive coronary angiography. At a threshold of a luminal narrowing of ≥ 50%, CCTA showed a higher sensitivity and lower specificity compared with stress echocardiography (100% vs 82.3% and 76.9% vs 88.4% for sensitivity and specificity, respectively). By increasing the threshold of the coronary stenosis to ≥ 70%, specificity was equal for both modalities (88.4%), whereas the sensitivity remained superior for CCTA when compared with echocardiography (100% vs 82.3%). The relatively early CT technology (64-slices) was associated with elevated settings (120kV and an effective tube current of 550 mA to 850mA) and hence higher radiation dose.40,41
SCIENTIFIC EVIDENCE IN THE SETTING OF STABLE CHEST PAINStable chest pain or stable angina is defined as chest tightness that worsens with exertion and improves with rest and is due to an imbalance between myocardial oxygen supply and demand.42 Patients with stable chest pain were included in the multicenter CORE64-study, which assessed the performance of CCTA and coronary artery calcium scan compared with invasive coronary angiography.43 Of 405 patients, CCTA showed a high diagnostic accuracy in detecting or ruling out stenosis of 50% or more in 291 patients as confirmed by subsequent invasive coronary angiography. The prevalence for CAD was 56%. Coronary computed tomography angiography had a sensitivity of 85%, a specificity of 90%, a positive predictive value of 91% and a negative predictive value of 83%. Values for the identification of patients having a subsequent revascularization were similar for CCTA with an area under the curve of 0.84, and an area under the curve of 0.82 for invasive angiography. Disease severity as defined by CCTA correlated very well with assessment by invasive coronary angiography (r=0.81). Radiation exposure was 13.8±1.2 mSv for men and 15.2±2.4 mSv for women.
The prospective multicenter and multivendor study led by Meijboom et al44 included 360 patients with acute and stable (n=233) angina symptoms referred for CCTA and invasive coronary angiography. The sensitivity of CCTA in detecting significant CAD was 99%, but its specificity was only 64% with a positive predictive value of 86% and a negative predictive value of 97%. On a per-segment level, the sensitivity dropped to 88%, while specificity increased to 90%, supporting the remarkable capacity of CCTA to exclude significant stenosis, as mentioned in the section outlining the scientific evidence in the setting of acute chest pain. Radiation exposure was between 15.5±2.2 mSv and 18.4±3.2 mSv.
The prospective multicenter ACCURACY trial evaluated patients with stable angina referred for invasive coronary angiography.45 In 230 evaluated patients, the procedure showed a sensitivity of 95%, specificity of 83%, a positive predictive value of 64%, and a negative predictive value of 99% in detecting stenosis ≥ 50%. On a per-vessel level, sensitivity dropped to 84%, whereas specificity increased to 90% and negative predictive value remained stable.
Patients with an intermediate probability of significant CAD were included In the OMCAS trial.46 The sensitivity of CCTA was 81.3%, its specificity was 93.3%, its positive predictive value was 91.6%, and negative predictive value was 84.7%. Unlike the above-mentioned trials, the vessel-based analysis showed no statistically significant decrease in sensitivity (P=.56), whereas the negative predictive value increased by 10.0% to 94.7%.
In the recent PROMISE-trial, 100 003 patients with symptoms suggestive of CAD were either assigned to anatomical evaluation using CCTA or to functional testing, such as exercise, electrocardiography, nuclear stress testing, or stress echocardiography.47 Endpoints in this study were defined as cardiac death, MI, hospitalization for unstable angina, major procedural complication, radiation exposure, and invasive coronary angiography performed in patients that did not show obstructive CAD. The likelihood of obstructive CAD was 53.3±21.4%. After a follow-up period of 2 years, cardiac death, MI, hospitalization for unstable angina or major procedural complication occurred in 3.3% of patients in the CCTA group and in 3.0% of patients who underwent functional testing (P=.75). On the other hand, CCTA was followed by fewer invasive coronary angiographies in patients showing no obstructive CAD compared with functional testing (3.4% vs 4.3%; P=.02), although more patients underwent an invasive coronary angiography within a period of 90 days after presenting in the ED (12.2% vs 8.1%). The conclusion of this study was that an initial CCTA for evaluating patients with suspected CAD did not improve the clinical outcome.
The retrospective study by Shreibati et al48 comparing CCTA with functional noninvasive modalities in a nonacute setting showed that the use of CCTA was associated with elevated subsequent invasive procedures, such as invasive coronary angiography, and hence higher medical expenses. Compared with the nonionizing modalities, CCTA was associated with a lower likelihood of rehospitalization for acute MI, whereas the likelihood of all-cause mortality was similar for all modalities within a period of 180 days.
In a yet not published study, Mark et al (personal communication) showed that although CCTA initially had lower costs than functional testing, its cumulative cost was not higher compared with that of functional testing.49
In a recent prospective multicenter SCOT-Heart trial, 4146 patients out of a total of 9849 patients with stable angina were randomly assigned to SOC plus CCTA or SOC alone. After six weeks CCTA reclassified the diagnosis of CAD in 27% of the patients. After 1.7 years CCTA diagnosed patients did not show a statistically-significant reduction in fatal and nonfatal MI. The median radiation dose in this trial was 4.1 mSv.
There are few studies of the cost effectiveness of CCTA in stable chest angina. Min et al50 showed in their ACCURACY-substudy that a CCTA strategy was more cost effective in the long-term with an incremental cost-effectiveness ratio of $ 20 429 per quality adjusted life-year compared with SPECT.
CONCLUSIONDue to technology developments in recent years, cardiovascular CT has become established as a robust modality in cardiovascular imaging. Recent technical advances include the use of low voltage settings, high-pitch acquisition avoiding oversampling, and the widespread applicability of IR algorithms. In parallel, there has been emerging scientific evidence of the clinical value of the techniques in the setting of acute chest pain in low-to-intermediate risk populations, as well as the diagnostic work-up of patients with stable chest pain syndrome. It can be anticipated that the increasing availability of advanced CT technology will further broaden the diffusion of the technique in the clinical work-up of cardiovascular patients.
CONFLICTS OF INTERESTNone declared.