The self-expanding Portico valve is a new transcatheter aortic valve system yielding promising preliminary results, yet there are no comparative data against earlier generation transcatheter aortic valve systems. The aim of this study was to compare the hemodynamic performance of the Portico and balloon-expandable SAPIEN XT valves in a case-matched study with echocardiographic core laboratory analysis.
MethodsTwenty-two patients underwent transcatheter aortic valve implantation with the Portico 23-mm valve and were matched for aortic annulus area and mean diameter measured by multidetector computed tomography, left ventricular ejection fraction, body surface area, and body mass index with 40 patients treated with the 23-mm SAPIEN XT. Mean aortic annulus diameters were 19.6±1.3mm by transthoracic echocardiography and 21.4±1.2mm by computed tomography, with no significant between-group differences. Doppler echocardiographic images were collected at baseline and at 1-month of follow-up and were analyzed in a central echocardiography core laboratory.
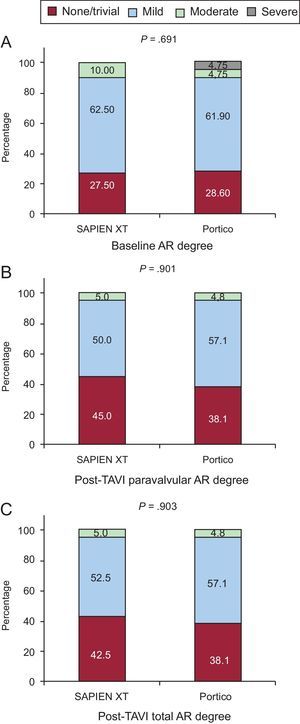
ResultsThere were no significant between-group differences in residual mean transaortic gradients (SAPIEN XT: 10.4±3.7mmHg; Portico: 9.8±1.1mmHg; P=.49) and effective orifice areas (SAPIEN XT: 1.36±0.27cm2; Portico, 1.37±.29cm2; P=.54). Rates of severe prosthesis-patient mismatch (effective orifice area<0.65cm2/m2) were similar (SAPIEN XT: 13.5%; Portico: 10.0%; P=.56). No between-group differences were found in the occurrence of moderate-severe paravalvular leaks (5.0% vs 4.8% of SAPIEN XT and Portico respectively; P=.90).
ConclusionsTranscatheter aortic valve implantation with the self-expanding Portico system yielded similar short-term hemodynamic performance compared with the balloon-expandable SAPIEN XT system for treating patients with severe aortic stenosis and small annuli. Further prospective studies with longer-term follow-up and in patients with larger aortic annuli are required.
Keywords
Transcatheter aortic valve implantation (TAVI) is well-established for treating patients with symptomatic severe aortic stenosis deemed at high or prohibitive risk for surgical aortic valve replacement.1 Moreover, TAVI has been associated with improved hemodynamic and clinical outcomes in patients with small aortic annuli, with a lower incidence of prosthesis-patient mismatch (PPM) and a nonsignificant increase in significant aortic regurgitation (AR) compared with surgical aortic valve replacement in this group of patients.2–4 However, data on the treatment of patients with small annuli has been mainly limited to the use of small (23mm) balloon-expandable transcatheter valves.
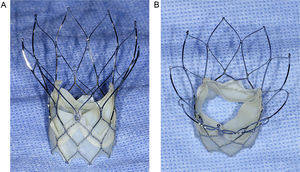
The Portico valve system (St. Jude Medical; Minneapolis, Minnesota, United States) is a second-generation transcatheter aortic valve consisting of a nitinol self-expanding frame containing 3 bovine pericardial leaflets and a porcine pericardial sealing cuff5 (Figure 1). The ability to retrieve and reposition the Portico valve represents 2 important positive features of this new valve system. The first available Portico valve was 23-mm size, and preliminary data in a small patient cohort demonstrated satisfactory clinical and hemodymamic outcomes in patients with small aortic annuli.6 However, it is well known that the lower amount of metal in the Portico stent frame, facilitating the ability to completely resheath a positioned valve, results in the production of lower radial forces compared with other self-expanding transcatheter aortic valve systems.7 This has raised concerns about how such changes may impact transcatheter valve performance (paravalvular leak rate and residual valve areas). In addition, the lower insertion of the valve leaflets within the stent frame, at the annular instead of supra-annular level, may also negatively impact valve hemodynamics. It would therefore be important to compare the hemodynamic performance of this valve with that of prior generation valves. Our objective was to compare the short-term hemodynamic performance of the 23-mm self-expanding Portico valve with the 23-mm balloon-expandable SAPIEN XT valve (SXTV) as evaluated in a case-matched population by a central echocardiography laboratory in patients with small aortic annuli.
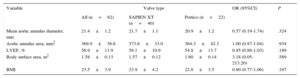
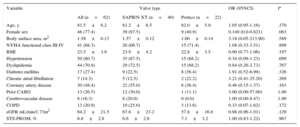
METHODSAcross 2 centers, 22 consecutive patients with severe symptomatic aortic stenosis underwent TAVI with the 23-mm self-expanding Portico valve. These patients were matched against 40 consecutive patients who had previously undergone TAVI with the 23-mm balloon expandable SXTV. Data from these patients had been prospectively acquired. The matching criteria (all pre-TAVI) involved: a) prosthesis size (23mm, exact match); b) aortic annulus area (within 50mm2) as assessed by multidetector computed tomography (MDCT); c) mean aortic annulus diameter as assessed by MDCT (within 0.5mm); d) left ventricular ejection fraction (within 10%) measured by transthoracic echocardiography; e) body surface area (within 0.4 m2), and f) body mass index (within 5kg/m2). A variable number of controls (from 1 to 4) were used, leading to a final sample of 40 matched patients who had undergone TAVI with the 23-mm SXTV. The values of the matched variables, according to valve type, are listed in Table 1.
Matched Variables (Each Group Received the 23-mm Prosthesis)
| Variable | Valve type | OR (95%CI) | P | ||
|---|---|---|---|---|---|
| All (n=62) | SAPIEN XT (n=40) | Portico (n=22) | |||
| Mean aortic annulus diameter, mm | 21.4±1.2 | 21.7±1.1 | 20.9±1.2 | 0.57 (0.19-1.74) | .324 |
| Aortic annulus area, mm2 | 369.9±36.6 | 373.6±33.0 | 364.3±42.3 | 1.00 (0.97-1.04) | .934 |
| LVEF, % | 56.9±11.9 | 58.1±10.9 | 54.8±13.7 | 0.95 (0.88-1.03) | .189 |
| Body surface area, m2 | 1.58±0.13 | 1.57±0.12 | 1.60±0.14 | 3.18 (0.05-213.20) | .589 |
| BMI | 23.5±3.9 | 23.9±4.2 | 22.8±3.5 | 0.90 (0.77-1.06) | .197 |
95%CI, 95% confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; OR, odds ratio.
Assessed by computed tomography.
Unless otherwise indicated, data are expressed as mean ± standard deviation.
Multidetector computed tomography examinations were performed and interpreted according to the criteria recommended by Achenbach et al.8 Briefly, the MDCT acquisition protocol was electrocardiogram gated (in systole), during suspended respiration, with a system of 64 simultaneously acquired slices and administration of iodinated contrast medium. Reconstruction of 0.6mm slice width throughout the entire imaging volume was obtained.
Prosthesis sizing was determined on the basis of aortic annulus measurements as previously described.5,9 The objective was to obtain a 1% to 15% prosthesis area oversizing with respect to the aortic annulus area in all patients. The TAVI procedure has been explained in detail in prior publications.1 The procedures were guided by fluoroscopy/angiography and transesophageal echocardiography. Procedural data and 30-day clinical events were prospectively recorded and defined according to the Valve Academic Research Consortium-2 (VARC-2) criteria.10 All TAVI procedures were performed under a compassionate clinical use program approved by Health Canada, and all patients provided signed informed consent.
All patients underwent a complete transthoracic echocardiographic examination, according to the guidelines of the American Society of Echocardiography,11,12 before the procedure and within 30 days post-TAVI. All echocardiographic examinations were centrally evaluated in the echocardiography core laboratory of the Quebec Heart and Lung Institute. All images were stored in digital format, and the analyses were performed off-line by experienced technicians and supervised by a cardiologist using an Image Arena Platform (TomTec Imaging Systems; Unterschleissheim, Germany). The following measurements were obtained for all patients: aortic annulus diameter, left ventricular outflow tract tract (LVOT) diameter, stroke volume, left ventricular ejection fraction evaluated using the biplane Simpson method, the mean and peak transvalvular gradient estimated with the modified Bernoulli formula, and valve effective orifice area (EOA) calculated using the continuity equation. The aortic annulus was measured in a zoomed parasternal long-axis view from the hinge point of the anterior aortic cusp and the ventricular septum to the junction of the posterior aortic cusp and the anterior mitral leaflet. After TAVI, LVOT diameter was measured just beneath the apical margin of the prosthesis stent.10,13,14 The LVOT Doppler recordings were also obtained just below the stent margin to ensure that the flow velocities were recorded at the same location as the LVOT.14,15 If the transcatheter valve was positioned low in the LVOT, with the stent margin below the apical end of the LVOT and protruding in the left ventricular cavity, the measurements of the LVOT diameter and velocity were obtained within the stent just below the transcatheter valve leaflets.16 The Doppler velocity index was calculated as the LVOT velocity/transvalvular velocity ratio.15,17 The EOA was indexed to the body surface area, and the presence of PPM was defined as an indexed EOA ≤ 0.85cm2/m2. A PPM was considered to be moderate if the indexed EOA was 0.65 cm2/m2 to 0.85cm2/m2, and severe if the indexed EOA was<0.65cm2/m2 .18
The presence, degree, and type (paravalvular or transvalvular) of AR were recorded in all patients. The AR severity was evaluated using a multiparametric approach and classified following the VARC-2 recommendations. The severity of AR was classified as follows: none-trace, mild, moderate, and severe. In the presence of paravalvular AR, the number of jets, localization, and the circumferential extent were also assessed. The circumferential extent of the paravalvular jets was measured in the parasternal short-axis views with color Doppler.14,15
Statistic AnalysisEach matched group was considered as a stratification variable. Conditional logistic regression was performed to detect association between valve type and selected variables observed in strata. The results were considered significant with P values<0.05.
Analyses were conducted using the statistical packages SAS, version 9.3 (SAS Institute Inc.; Cary, North Carolina, United States).
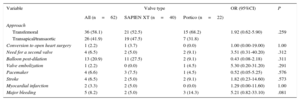
RESULTSThere were no significant between-group differences in baseline clinical characteristics, but there was a trend towards a higher prevalence of women in the SXTV group (Table 2). The main procedural and in-hospital events post-TAVI are listed in Table 3. There were no significant between-group differences in periprocedural events, although there was a nonsignificantly greater incidence of major bleeding in the Portico group compared with the SXTV group (14.3% vs 5.0%; P=.081). The rate of pacemaker implantation was low (< 10%) in the 2 groups. Two patients in the Portico group required second valve implantation. In 1 patient, severe AR was observed after first valve deployment. In another patient, the Portico valve embolized into the ascending aorta during the snaring maneuvers for valve repositioning, a second valve was successfully implanted. In a third patient, the valve moved toward the LVOT at the end of the deployment and it was implanted slightly more ventricularly than expected; however, no significant AR or hemodynamic repercussions were observed. In the same way, 2 patients treated with the SXTV required a second valve because of severe AR after first valve deployment that persisted following balloon post-dilatation. One patient in the SXTV group had a ventricular perforation (wire-related) requiring conversion to open surgery.
Baseline Characteristics, Overall and According to Transcatheter Valve Type
| Variable | Valve type | OR (95%CI) | P | ||
|---|---|---|---|---|---|
| All (n=62) | SAPIEN XT (n=40) | Portico (n=22) | |||
| Age, y | 81.5±6.2 | 81.2±6.5 | 82.0±5.9 | 1.05 (0.95-1.16) | .370 |
| Female sex | 48 (77.4) | 39 (97.5) | 9 (40.9) | 0.149 (0.0-0.821) | .063 |
| Body surface area, m2 | 1.58±0.13 | 1.57±0.12 | 1.60±0.14 | 3.18 (0.05-213.90) | .589 |
| NYHA functional class III-IV | 41 (68.3) | 26 (66.7) | 15 (71.4) | 1.08 (0.33-3.51) | .899 |
| BMI | 23.5±3.9 | 23.9±4.2 | 22.8±3.5 | 0.90 (0.77-1.06) | .197 |
| Hypertension | 50 (80.7) | 35 (87.5) | 15 (68.2) | 0.34 (0.09-1.23) | .099 |
| Dyslipidemia | 44 (70.9) | 29 (72.5) | 15 (68.2) | 0.84 (0.26-2.73) | .767 |
| Diabetes mellitus | 17 (27.4) | 9 (22.5) | 8 (36.4) | 1.91 (0.52-6.99) | .326 |
| Chronic atrial fibrillation | 7 (14.3) | 5 (12.5) | 2 (22.2) | 3.21 (0.41-25.20) | .269 |
| Coronary artery disease | 30 (48.4) | 22 (55.0) | 8 (36.4) | 0.46 (0.15-1.37) | .163 |
| Prior CABG | 13 (26.5) | 12 (30.0) | 1 (11.1) | 3.00 (0.00-57.00) | 1.00 |
| Cerebrovascular disease | 8 (16.3) | 8 (20.0) | 0 (0.0) | 1.00 (0.00-8.47) | 1.00 |
| COPD | 13 (20.9) | 10 (25.0) | 3 (13.6) | 0.33 (0.07-1.62) | .172 |
| eGFR mL/min/1.73m2 | 64.2±21.5 | 67.8±23.2 | 57.8±16.8 | 0.98 (0.96-1.01) | .129 |
| STS-PROM, % | 6.8±2.8 | 6.6±2.6 | 7.1±3.2 | 1.00 (0.83-1.22) | .967 |
95%CI, 95% confidence interval; BMI, body mass index; CABG, coronary arterial bypass graft; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; OR, odds ratio; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
Post-procedural Complications According to Transcatheter Valve Type
| Variable | Valve type | OR (95%CI) | P | ||
|---|---|---|---|---|---|
| All (n=62) | SAPIEN XT (n=40) | Portico (n=22) | |||
| Approach | |||||
| Transfemoral | 36 (58.1) | 21 (52.5) | 15 (68.2) | 1.92 (0.62-5.90) | .259 |
| Transapical/transaortic | 26 (41.9) | 19 (47.5) | 7 (31.8) | ||
| Conversion to open heart surgery | 1 (2.2) | 1 (3.7) | 0 (0.0) | 1.00 (0.00-19.00) | 1.00 |
| Need for a second valve | 4 (6.5) | 2 (5.0) | 2 (9.1) | 3.51 (0.31-40.20) | .312 |
| Balloon post-dilation | 13 (20.9) | 11 (27.5) | 2 (9.1) | 0.43 (0.08-2.18) | .311 |
| Valve embolization | 1 (2.2) | 0 (0.0) | 1 (4.5) | 5.30 (0.20-31.20) | .291 |
| Pacemaker | 4 (6.6) | 3 (7.5) | 1 (4.5) | 0.52 (0.05-5.25) | .576 |
| Stroke | 4 (6.5) | 2 (5.0) | 2 (9.1) | 1.82 (0.23-14.60) | .573 |
| Myocardial infarction | 2 (3.3) | 2 (5.0) | 0 (0.0) | 1.29 (0.00-11.60) | 1.00 |
| Major bleeding | 5 (8.2) | 2 (5.0) | 3 (14.3) | 5.21 (0.82-33.10) | .081 |
95%CI, 95% confidence interval; OR, odds ratio.
Unless otherwise indicated, data are expressed as No. (%).
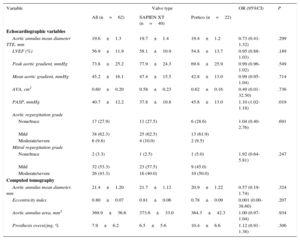
The main echocardiography and MDCT characteristics according to valve type are listed in Table 4. There were no between-group differences in the severity of aortic stenosis (P>.70 for mean transvalvular gradient and aortic valve area), and aortic annulus diameter (as evaluated by transthoracic echocardiography) was also similar between the 2 groups (P=.30). Apart from the matched MDCT variables (aortic annulus area and mean diameter), the 2 groups were also well balanced with regards to the aortic annulus eccentricity index (P=.21). Also, the degree of valve oversizing was similar in the Portico and SXTV groups (10.4±6.6% vs 6.5±5.6%; P=.31).
Echocardiography and Computed Tomography Data at Baseline, Overall and According to Valve Type
| Variable | Valve type | OR (95%CI) | P | ||
|---|---|---|---|---|---|
| All (n=62) | SAPIEN XT (n=40) | Portico (n=22) | |||
| Echocardiographic variables | |||||
| Aortic annulus mean diameter TTE, mm | 19.6±1.3 | 19.7±1.4 | 19.4±1.2 | 0.73 (0.41-1.32) | .299 |
| LVEF (%) | 56.9±11.9 | 58.1±10.9 | 54.8±13.7 | 0.95 (0.88-1.03) | .189 |
| Peak aortic gradient, mmHg | 73.8±25.2 | 77.9±24.3 | 69.8±25.9 | 0.99 (0.96-1.02) | .549 |
| Mean aortic gradient, mmHg | 45.2±16.1 | 47.4±15.5 | 42.8±13.0 | 0.99 (0.95-1.04) | .714 |
| AVA, cm2 | 0.60±0.20 | 0.58±0.23 | 0.62±0.16 | 0.49 (0.01-32.50) | .736 |
| PASP, mmHg | 40.7±12.2 | 37.8±10.8 | 45.8±13.0 | 1.10 (1.02-1.18) | .019 |
| Aortic regurgitation grade | |||||
| None/trace | 17 (27.9) | 11 (27.5) | 6 (28.6) | 1.04 (0.40-2.76) | .691 |
| Mild | 38 (62.3) | 25 (62.5) | 13 (61.9) | ||
| Moderate/severe | 6 (9.8) | 4 (10.0) | 2 (9.5) | ||
| Mitral regurgitation grade | |||||
| None/trace | 2 (3.3) | 1 (2.5) | 1 (5.0) | 1.92 (0.64-5.81) | .247 |
| Mild | 32 (53.3) | 23 (57.5) | 9 (45.0) | ||
| Moderate/severe | 26 (43.3) | 16 (40.0) | 10 (50.0) | ||
| Computed tomography | |||||
| Aortic annulus mean diameter, mm | 21.4±1.20 | 21.7±1.12 | 20.9±1.22 | 0.57 (0.19-1.74) | .324 |
| Eccentricity index | 0.80±0.07 | 0.81±0.06 | 0.78±0.09 | 0.001 (0.00-38.60) | .207 |
| Aortic annulus area, mm2 | 369.9±36.6 | 373.6±33.0 | 364.3±42.3 | 1.00 (0.97-1.04) | .934 |
| Prosthesis oversizing, % | 7.9±6.2 | 6.5±5.6 | 10.4±6.6 | 1.12 (0.91-1.38) | .306 |
95%CI, 95% confidence interval; AVA, aortic valve area; LVEF, left ventricular ejection fraction; OR, odds ratio; PASP, pulmonary artery systolic pressure; TTE, transthoracic echocardiography.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
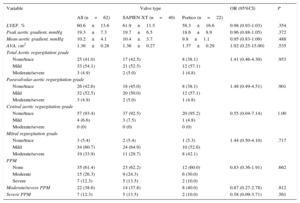
Echocardiographic data post-TAVI, according to valve type, are listed in Table 5. Left ventricular outflow tract measurements of diameter and velocity were performed prestent in all patients, according the recommendations of VARC-2. The overall mean transprosthetic gradient decreased from 45.2±16.1mmHg to 10.2±4.1mmHg (P<.001), and the mean EOA increased from 0.60±0.2cm2 to 1.36±0.28cm2 (P<.001) post-TAVI. There were no between-group differences in residual transaortic mean gradients (P=.49) and EOAs (P=.54). No between-group differences were found in the presence and severity of paravalvular leaks (P=.90) or total AR (P=.95) (Figures 2 and 3).
Echocardiography Data Post-transcatheter Aortic Valve Implantation According to Valve Type
| Variable | Valve type | OR (95%CI) | P | ||
|---|---|---|---|---|---|
| All (n=62) | SAPIEN XT (n=40) | Portico (n=22) | |||
| LVEF, % | 60.6±13.6 | 61.9±11.5 | 58.3±16.6 | 0.98 (0.93-1.03) | .354 |
| Peak aortic gradient, mmHg | 19.3±7.3 | 19.7±6.5 | 18.6±8.9 | 0.96 (0.88-1.05) | .372 |
| Mean aortic gradient, mmHg | 10.2±4.1 | 10.4±3.7 | 9.8±1.1 | 0.95 (0.83-1.09) | .488 |
| AVA, cm2 | 1.36±0.28 | 1.36±0.27 | 1.37±0.29 | 1.92 (0.25-15.00) | .535 |
| Total Aortic regurgitation grade | |||||
| None/trace | 25 (41.0) | 17 (42.5) | 8 (38.1) | 1.41 (0.46-4.30) | .953 |
| Mild | 33 (54.1) | 21 (52.5) | 12 (57.1) | ||
| Moderate/severe | 3 (4.9) | 2 (5.0) | 1 (4.8) | ||
| Paravalvular aortic regurgitation grade | |||||
| None/trace | 26 (42.6) | 18 (45.0) | 8 (38.1) | 1.48 (0.49-4.51) | .901 |
| Mild | 32 (52.5) | 20 (50.0) | 12 (57.1) | ||
| Moderate/severe | 3 (4.9) | 2 (5.0) | 1 (4.8) | ||
| Central aortic regurgitation grade | |||||
| None/trace | 57 (93.4) | 37 (92.5) | 20 (95.2) | 0.55 (0.04-7.14) | 1.00 |
| Mild | 4 (6.6) | 3 (7.5) | 1 (4.8) | ||
| Moderate/severe | 0 (0) | 0 (0) | 0 (0) | ||
| Mitral regurgitation grade | |||||
| None/trace | 3 (5.4) | 2 (5.4) | 1 (5.3) | 1.44 (0.50-4.10) | .717 |
| Mild | 34 (60.7) | 24 (64.9) | 10 (52.6) | ||
| Moderate/severe | 19 (33.9) | 11 (29.7) | 8 (42.1) | ||
| PPM | |||||
| None | 35 (61.4) | 23 (62.2) | 12 (60.0) | 0.83 (0.36-1.91) | .662 |
| Moderate | 15 (26.3) | 9 (24.3) | 6 (30.0) | ||
| Severe | 7 (12.3) | 5 (13.5) | 2 (10.0) | ||
| Moderate/severe PPM | 22 (38.6) | 14 (37.8) | 8 (40.0) | 0.87 (0.27-2.78) | .812 |
| Severe PPM | 7 (12.3) | 5 (13.5) | 2 (10.0) | 0.58 (0.09-3.71) | .561 |
95%CI, 95% confidence interval; AVA, aortic valve area; LVEF, left ventricular ejection fraction; OR, odds ratio; PPM, patient prosthesis mismatch.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
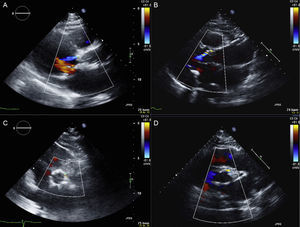
Postprocedural echocardiographic images of 2 patients who had undergone transcatheter aortic valve implantation with the Portico valve (A and C) and the SAPIEN XT valve (B and D). Mild paravalvular leak after Portico valve implantation (A: long axis; C: short axis) and SAPIEN XT valve implantation (B: long axis; D: short axis).
This first report comparing the hemodynamic performance of the newer 23-mm self-expanding Portico valve with the 23-mm balloon-expandable SXTV for treating patients with severe aortic stenosis and small aortic annuli highlights similar hemodynamic performances for the 2 valve systems, with mean residual gradients<10mmHg and rates of severe PPM<15%. In addition, the rate of moderate or severe paravalvular leaks was ∼5% in the groups.
Some, but not all, previous studies demonstrated higher earlier paravalvular leak rates with the use of the self-expanding CoreValve system.19–22 Although long-term differences between valves are uncertain, this higher earlier paravalvular leak incidence was partially attributed to a lower achieved radial force as compared with the balloon-expandable Edwards system.23 Subsequent concerns were therefore raised about the newer Portico self-expanding transcatheter aortic valve system, largely due to its reduced amount of metal and cell size of the stent frame also resulting in a lower radial force akin to the CoreValve system.7 These design features were incorporated with the objective of producing a fully retrievable and repositionable transcatheter aortic valve system in the advent of valve malpositioning or embolization. The present report confirms that these design features did not translate into a greater occurrence and heightened severity of paravalvular leaks. The larger cell area design results in a high tissue to frame ratio at the valve cuff segment, which has been proposed as a potential mechanism of reducing AR by allowing valve tissue to conform around calcific nodules at the annular level. Furthermore, it is now well accepted that proper device positioning is a key factor related to the occurrence of AR.24 In this regard, the repositionable-retrievable nature of the Portico transcatheter aortic system may contribute to an improved final positioning of the valve.
The occurrence of PPM post-TAVI (and surgical aortic valve replacement) remains a major concern following the treatment of patients with severe aortic stenosis and small annuli. Two recent substudies of the PARTNER trial,2,3 demonstrated lower PPM rates post-balloon-expandable TAVI compared with surgical aortic valve replacement in such patients. In addition to the lower radial force imparted by the Portico valve, the fact that the valve leaflets are positioned very low (at the annular instead of supra-annular level) within the stent frame could have translated into increased residual gradients and higher PPM rates. The present study outlined a mean residual gradient of<10mmHg and a 40% moderate or severe PPM rate following Portico valve implantation in patients with small annuli, similar to the results obtained with the balloon-expandable Edwards system. This rate is also in accordance with the PPM rate reported in prior studies in patients with small annuli.3,4 The adaptability and normal valve functioning of the Portico system has been proven in circular and noncircular structures in bench testing (unpublished data) and this likely assists in maintaining the low residual gradients despite the lower radial forces. Future studies are needed to evaluate how this system compares with other TAVI systems with supra-annular valvular function properties (ie, Medtronic CoreValve system).
St. Jude Medical temporarily halted the Portico valve program in September 2014 following the detection of reduced valve leaflet mobility as evaluated by 4-dimensional MDCT among patients participating in the United States pivotal trial (ClinicalTrials.gov: 02000115). This decision was undertaken even though preliminary clinical and echocardiography data failed to suggest any issues with the valve system. The present study confirms that early valve hemodynamics of the Portico system are comparable to contemporary TAVI systems. Importantly, a lack of increased residual gradients and the absence of cases of significant transvalvular AR were observed. Unfortunately, no transesophageal echocardiography or contrast MDCT exams were performed at follow-up in our study population, and no additional data to current knowledge on valve leaflet motion can be provided.
Although this study was not powered to detect differences in clinical events, no significant differences were found in early events between groups. No cases of valve thrombosis were reported, which is consistent with the previously reported low incidence of this complication.25 Of note, the permanent pacemaker implantation rate was low in both groups, particularly in the Portico group (4.8%). This incidence is much lower than contemporary data from other self-expanding transcatheter aortic valves,26 and may be partially due to the specific design of the Portico valve system. Compared with the CoreValve system, the Portico valve does not contain a flared inflow and it presents leaflets and a tissue cuff located low on the support frame, thereby minimizing device protrusion into the LVOT. In addition, deep valve implantation has been reported as an independent factor predicting the need for permanent pacemaker implantation post-TAVI with self-expanding valves.26 Due to the ability to completely resheath, the Portico valve can be repositioned in order to avoid lower implantation.
LimitationsSeveral caveats of the present analysis warrant further consideration, including its nonrandomized design and limited sample size. This was partially compensated by a strict matching process between groups, including 3-dimensional MDCT data, and a uniform standardized analysis performed by a central echocardiography core laboratory. However, these results need to be confirmed by a larger, prospectively designed randomized trial. As expected when evaluating a new transcatheter heart valve, both centers had less experience with the Portico valve compared with their experience of SXTV. Only patients who survived the hospitalization period were included in the present analysis, and, as a result, there may have been a possible “positive” patient selection bias in both groups. Also, no systematic data on calcium burden at the valve-annulus level (a factor that may influence the incidence of paravalvular leaks) were obtained in this study, precluding the use of this variable for the matching process. These data refer to tricuspid aortic valves, future studies in bicuspid aortic stenosis are needed.27 Finally, these data apply exclusively to the 23-mm valve and cannot be extrapolated to larger aortic annuli and valve sizes.
CONCLUSIONSThe 23-mm self-expanding Portico valve system was associated with a similar hemodynamic performance to the balloon-expandable SXTV system. While we await the results of the prospective randomized United States pivotal trial, the present report suggest that the Portico valve system could be a valid alternative for treating patients with severe aortic stenosis and small aortic annuli.
FUNDINGM. Del Trigo and O. Abdul-Jawad Altisent are supported by a research grant from the Fundación Alfonso Martín Escudero (Spain).
CONFLICTS OF INTERESTJ. Rodés-Cabau has received research grants from Edwards Lifesciences and St. Jude Medical. J. Webb has received consulting fees from Edwards Lifesciences and St. Jude Medical. P. Pibarot has received research grants from Edwards Lifesciences.