With the rapidly rising number of patients surviving cancer, often in the setting of new or pre-existing cardiovascular disease and risk factors, a need has arisen for a specialty within the realm of cardiovascular care that can evaluate and manage these patients along with our colleagues in oncology and hematology. By the same token, all health care providers involved in the care of cancer patients with heart disease must be fully aware of the impact of adverse cardiovascular effects on the survival of these patients. Collaboration is required to mitigate the effect of cardiovascular toxicity associated with these necessary life-saving cancer therapies. The cardio-oncologist plays a pivotal role in bridging the 2 specialties, by creating a comprehensive plan to address the comorbidities as well as to provide guidance on the optimal choice of therapy. In this 3-part review, we will outline: a) the significant impact of cancer therapies on the cardiovascular health of patients with cancer and cancer survivors, b) the advantage of a multidisciplinary team in addressing these cardiovascular complications, and c) the delivery of clinical care to patients with cancer and heart disease.
Keywords
Cancer and cardiovascular diseases (CVDs) may coexist in a patient due to a common occurrence of risk factors and aging,1 and there is also a growing evidence of a higher prevalence of CVDs in patients diagnosed with cancer.2 In addition, cancer therapies can have a myriad of effects on the cardiovascular (CV) system, depending on the type of therapy. Furthermore, a patient with cancer and pre-existing CVDs who undergoes cancer therapy is at increased risk for the development of cardiotoxicity.3 Cardiovascular complications have been reported to profoundly impact quality of life and survival of patients with cancer,4,5 implying that their recognition and early management must become an important element in the overall care for cancer patients.6–8
A new discipline termed “cardio-oncology” has thus evolved to address the CV needs of cancer patients and optimize their care in a multidisciplinary approach. This new field is committed to optimally manage CV adverse effects of cancer therapy as well as to assist in the overall care of cancer patients from the initial assessment to survivorship.6
The present review outlines the significant impact of cancer therapies on the CV health of patients with cancer and cancer survivors as well as the advantage of a comprehensive cardio-oncology service in addressing CV toxicity and delivering clinical care to patients with cancer and heart disease.
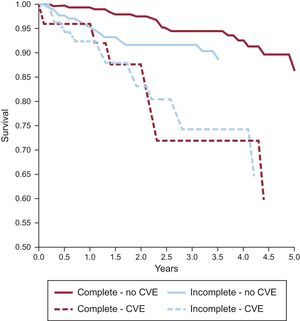
DEFINING THE RISKThere has been a remarkable improvement in the care of patients with cancer over the past 2 decades. A combination of early cancer diagnosis, use of novel targeted therapies, radiation therapy, and more radical surgical techniques has decreased cancer-related mortality.9,10 There is an estimated 14.5 million long-term adult and pediatric cancer survivors in the United States, and this number is expected to reach 19 million by the year 2024.9,11,12 However, effective cancer therapies can result in short- and long-term CV complications that can compromise their clinical benefits by impacting quality of life and survival,5,6,9,10 as shown in Figure 1.5 Indeed, the risk of CV death in some tumor groups may exceed that of tumor recurrence for many forms of cancer.13,14
Cardiovascular events (CVE) in patients receiving complete or incomplete trastuzumab treatment demonstrating that patients with CVE have worse survival regardless of trastuzumab completion status. Reproduced from Wang et al.5 with permission.
The entire CV system can be affected by cancer therapies, although cardiotoxicity is mostly defined based on changes in left ventricular (LV) ejection fraction.10,15 The spectrum of adverse CV effects of cancer therapies includes LV dysfunction and heart failure (HF), acute coronary syndromes, hypertension, rhythm disturbances, thromboembolic events, valvular disease, and pericardial disease (Figure 2).16
An overview of the cardiovascular adverse effects of chemotherapy and radiation. HER2, human epidermal growth factor receptor 2. Reproduced from Lenneman et al.16 with permission.
Left ventricular dysfunction and HF are the most frequent manifestations. The incidence of LV dysfunction and HF ranges from 5% to 25% in patients treated with anthracyclines17,18; from 2% to 33% with vascular endothelial growth factor (VEGF) inhibitor therapy,16,19 and in patients treated with HER2 (human epidermal growth factor receptor 2)-targeted therapies around 2.5% for HF and 11.2% for LV dysfunction.20 Anthracycline-induced cardiomyopathy is often irreversible if not identified early, as the prompt initiation of standard HF therapy is one of the critical factors for its recovery.16,17,21 It can lead to progressive end-stage HF with a prognosis that is worse than that for ischemic or dilated cardiomyopathies and even possibly worse than for cancer recurrence.6,22,23
Systemic hypertension (new-onset or worsening) has also emerged as a frequent adverse effect associated with VEGF-inhibitors. The incidence of hypertension ranges from 19.1% to 44.4%, with the lowest incidence seen in patients treated with sorafenib and the highest incidence observed with regorafenib.24 High-grade (grade 3 or 4) hypertension secondary to axitinib therapy was reported to be associated with significant morbidity, and might result in the need for a dose reduction or discontinuation of this medication.25
Arrhythmias, especially bradyarrhythmias, are the most commonly observed toxicity due to microtubule inhibitors, such as docetaxel and paclitaxel.26 Asymptomatic and self-limited bradycardia has occurred in up to 29% of patients.26 In comparison, severe arrhythmias including supraventricular and ventricular tachyarrhythmia are rare (incidence of 0.24% and 0.26%, respectively) and are usually self-limited as well.16,26–28 The risk of QT prolongation varies with different drugs, with arsenic trioxide being the most relevant. This drug prolongs the QT interval in 26% to 93% of patients, and life-threatening arrhythmias such as torsade de pointes have been reported not infrequently.29 Among other cancer therapies, the tyrosine kinase inhibitors, and specifically vandetanib, has the second highest incidence of QT prolongation.30–32
Acute coronary syndrome can include the entire spectrum from unstable angina to acute myocardial infarction and even sudden cardiac death. It is a rare but potentially serious adverse effect of some cancer therapies.24 Premature acute coronary syndrome is one of the most concerning late consequences of cisplatin-based chemotherapy, and occurs in 5.6% to 6.7% patients, with a 3-fold higher relative risk than in aged-matched controls.16,33 In contrast, cardiac ischemia is a short-term complication related to the antimetabolite 5-fluorouracil as well as with VEGF-inhibitors, with an incidence ranging from 1.5% to 2%.34–36
The risk of CV complications of cancer treatments is enhanced by additional patient-and treatment related factors including underlying CV diseases, combination therapy and prior radiotherapy.6 For instance, Armstrong et al.37 reported that CV risk factors, particularly hypertension, significantly increased the risk for coronary artery disease (relative risk [RR], 6.1), HF (RR, 19.4), valvular disease (RR, 13.6), and arrhythmia (RR, 6.0) among survivors who received chest-directed radiotherapy or anthracycline chemotherapy. Additionally, the risk for each cardiac event increased with increasing number of CV risk factors.
All together, the data suggest that CV complications are common and of clinical significance. This warrants awareness from both oncologists/hematologists and cardiologists of the potential cardiac hazards from cancer therapy, especially in patients with recognized risk factors, as well as of the need for early diagnosis and prompt management of CV risk factors and/or complications to avoid or minimize the risk of potentially serious cardiac events in cancer patients and survivors.
MULTIDISCIPLINARY CARDIO-ONCOLOGY PROGRAMThe medical community is becoming increasingly aware of CV concerns in cancer patients and survivors, as highlighted recently in both a national38 and in an international cardiac oncology survey.39 However, the international survey also demonstrated wide variation in clinical practice between cardiologists and oncologists regarding the diagnosis, management, and monitoring of oncology patients at risk of CV complications as well as the appropriate clinical management of cancer patients who develop cancer therapeutics-related cardiac dysfunction (CTRCD). For example, in a case of trastuzumab-induced CTRCD, oncologists were more likely to “discontinue trastuzumab, resume if ejection fraction normalizes” whereas cardiologists were more willing to “discontinue trastuzumab permanently”, thus depriving these patients of potentially life-saving therapy.39 Additionally, in routine clinical practice of cancer survivors, there is poor adherence to cardiac surveillance and guideline-recommended HF therapy. Unfortunately cardiac dysfunction is diagnosed late usually in the setting of signs and symptoms of HF and is inappropriately treated.40 Consequently, a multidisciplinary comprehensive approach that links both cardiology and oncology expertise in a collaborative effort is critical to optimize patient outcomes.8,41–44
Benefits of Multidisciplinary Cardio-Oncology CareMultidisciplinary teams should improve coordination, communication, and decision-making between health care team members and patients, and help improve outcomes.45 The referral process for front-line health providers can be facilitated through a common access point such as electronic medical records. This, in conjunction with an ease of referral, should expedite patient assessment as well as cardiac surveillance and improve patient satisfaction.46 Group meetings between members with multiple areas of expertise relevant to cardio-oncology would reduce knowledge gaps and improve consistency in patient care.46,47 This is especially important to overcome, since currently there is a lack of guidelines for the CV management of patients with cancer, a history of cancer and management of CV complications from cancer therapy. Only a group consensus from the American Society of Echocardiography and the European Association of Cardiovascular Imaging15 is available and just recently in 2016 the Canadian Cardiovascular Society published its society guidelines for evaluation and management of CV complications of cancer therapy.31 Additionally, there is evidence that interdisciplinary patient care including cardiology consultation in the management of patients with CTRCD40 as well as in other HF populations48,49 improves outcomes. Cardiology care was associated with higher rates of guideline-recommended HF therapy40,48,49 and better survival40,48 compared with care by physicians from other disciplines. There is also recent evidence that referral to a cardio-oncology clinic is associated with a higher rate of completion of cancer therapy, which might improve patient outcomes by increasing the likelihood of cancer remission.46,50
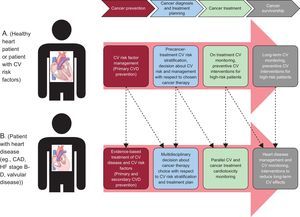
GoalsCardio-oncology is a medical subspecialty dedicated to providing comprehensive CV care to cancer patients from cancer diagnosis to survivorship. The primary focus is to support cancer patients through CV risk stratification, CV monitoring during and after cancer treatments, as well as treatment of pre-existing and newly diagnosed CV disease. The key element for high-risk patients is preventive measures depending on the associated risk factors in order to preserve CV health. In those with heart disease a multidisciplinary approach to cancer therapy choice would be most appropriate to minimize cardiotoxicity31,38,46 (Figure 3).38 Prevention and management strategies of cardiotoxicity will be important to allow the optimal cancer therapy while protecting CV health, and thus to improve both the cardiological and oncological outcomes.1,8,38,46
An example of the continuum of cardiovascular care on a timeline of cancer diagnosis, treatment, and survivorship. Patient A represents a patient with no existing cardiovascular disease and patient B represents a patient with a pre-existing cardiovascular condition. CAD, coronary artery disease; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure. Reproduced from Barac et al.38 with permission.
Other goals that cardio-oncology should explore are the design of innovative strategies to diagnose and prevent cardiotoxicity and the development of evidence-based guidelines, ie, standardize the diagnosis, management, and monitoring of cardiotoxicity.50,51 Indeed, research efforts are underway to develop practical cardiac risk stratification tools, to better select who may benefit from more intensive cardiac monitoring during cancer therapy and follow-up, identify the best strategy in the early identification and treatment of cardiotoxicity to avoid long-term sequelae,52–54 and identify long-term cardiac consequences of these therapies in cancer survivors and improve cardiac surveillance in this population.50
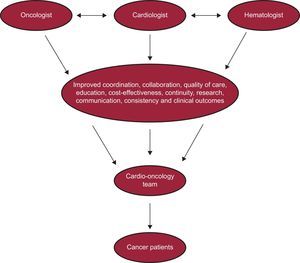
To achieve this goal, there needs to be an organized collaboration and continued communication between the different stakeholders involved in the care of cancer patients to share expertise and resposibilities8,55,56 (Figure 4).57
Cardio-oncology multidisciplinary team. Reproduced from Barros-Gomes et al.57 with permission.
A multidisciplinary cardiac oncology clinic requires the participation of many types of health care providers including cardiologists, hematologist-oncologists, radiation oncologists, surgical oncologists, and clinical support staff, such as medical assistants, nurses, and physician extenders (nurse practitioners/physician assistants). Depending on the clinical scenario, dietitians, pharmacists, psychosocial providers, exercise physiologists, palliative care specialists, and primary care providers might be required.8,46,58
All stakeholders should have a common platform of communication, which can be provided by the electronic medical record. While the hematologist/oncologist will deliver the most up-to-date treatment, the cardiologist ideally needs to have additional expertise in HF and/or CV imaging to make decisions based on history, physical examination, and high-quality images to ensure the best treatment is provided, and he/she is expected to offer advice on management strategies to best ensure cardiac safety.8,58,59 The clinical support staff is essential as in any other clinic to schedule all the necessary appointments, which include–but are not limited to–chemotherapy, radiology studies, psychosocial, dietitians, cardio-oncology, oncology, and surgical appointments, etc.; patient education and engagement; and for more stable patients nurse practitioners or physician assistants could follow up the patients, freeing up some time for the cardio-oncologist to incorporate more complex patients into their clinical schedule.58
Ideally each team member should meet with the patient during the initial encounter to identify care needs and review treatment strategies. Physical, mental and social conditions should be taken into account when formulating the treatment strategy to pursue. This team approach will allow any new concern to be directed to the appropriate member for management. This integral approach will benefit not only the patient but also improve the efficiency of the cardio-oncology service.46
Current SituationWith growing patient need, a number of cardio-oncology clinical programs are emerging mainly in academic centers and have been added to the few pioneer dedicated cardio-oncology programs, including the MD Anderson Cancer Center, Memorial Sloan Kettering Cancer Center, Vanderbilt-Ingram Cancer Center, University of Pennsylvania Abramson Cancer Center, Mayo Clinic, and Dana-Farber Cancer Institute. In the United States, 27% of cardio-oncology services are offered by a multidisciplinary team according to a recent national cardiac survey; the main cause of referral is for preoperative consultation (35%). In 16% of cases, cardiac consultation is provided by a single cardiologist with expertise in cardio-oncology.38 In an international survey of health care providers involved in the management of cancer patients exposed to cardiotoxic therapy, a dedicated cardio-oncology clinic was available in 54% of academic centers, and in 29% in community hospitals.39 At our institution, the cardio-oncology practice was initially established in 2013 through electronic-based consultations (“e-consults”). These types of consultations emerged as a mechanism to provide efficient clinical care in a timely manner through the electronic medical record without face-to-face interaction.57 With the growth of the complexity of our cancer population, face-to-face consultations were added and became the main mode of service. A dedicated cardio-oncology clinic was then started in 2014 and has had significant growth in the past last 3 years.
By the same token, it is important to incorporate quality standards as proposed by the Spanish Society of Cardiology for HF clinics, which could be applied to these units as they are becoming more and more an integral part in today's clinical practice. By doing so, patients will benefit from a similar approach to diagnosis and management across cardio-oncology centers, facilitating the accreditation process and interinstitutional research that should be implemented in the near future.60
There is no question that cardio-oncology is a rapidly evolving area of medicine. This is reflected but not limited by the increased awareness of the medical community of the importance of CV care in patients with cancer, the exponentially increasing publications about cardio-oncology, the recognition of the specialty by the American College of Cardiology in 2015, the development of Cardio-Oncology Journals and reviews, and the growing number of international conferences dedicated to this specialty.8,38
CARDIOVASCULAR CLINICAL CARE OF CANCER PATIENTS AND CANCER SURVIVORSA multidisciplinary approach incorporating cardiology and oncology expertise is needed for the CV evaluation of patients before, during, and after cancer therapy.
Identifying the High-risk PopulationBefore the initiation of cancer therapy, patients should be stratified in their baseline risk for cardiotoxicity, which allows cardiologists to better assist oncologists to individualize therapy and define the type of cardiac monitoring that should be followed.15 For patients at low risk, cardiac monitoring may not be necessary, allowing health care resources to be allocated to higher-risk individuals who should be considered for a more aggressive surveillance strategy.31 In general, patients with established or risk factors for CV disease, increasing age, and exposure to combination cancer therapy are considered to be at high risk for the development of cardiotoxicity.31 Several risk models have been used to predict cardiotoxicity from cancer therapy.31 We proposed a cardiotoxicity risk score that includes both drug-related risk and patient-related risk factors.6 Elements such as age (< 15 or > 65 years), female sex, history of cardiomyopathy and HF, coronary disease, hypertension, diabetes, prior or concurrent anthracycline, and prior or concurrent chest radiation have been used to identify at risk patients. In addition to a complete history and physical examination, diagnostic tests such as electrocardiography, echocardiography with strain imaging, and troponin measurement are recommended during baseline cardiac assessment and follow-up.15 Ideally, this assessment should be performed in all patients who will undergo potentially cardiotoxic treatment regimens, or at least in those considered to be at high risk for the development of cardiotoxicity.15,61–63
Detection and Prevention of CardiotoxicityPatients are usually serially followed up for evidence of LV dysfunction (ie, CTRCD defined as a decrease in LV ejection fraction > 10% to an absolute value less than 53%) or subclinical LV dysfunction (abnormal global longitudinal strain). Although not routinely used in clinical practice, cardiac biomarkers (ie, troponin, B-type natriuretic peptide) are a reliable diagnostic tool for the early identification and monitoring of cardiotoxicity.31,64 Baseline and periodic electrocardiograms are useful for cancer therapies associated with increased risk of arrhythmia. Blood pressure monitoring is recommended during VEGF-inhibitors.31
There are currently 2 position papers on the recommendations for the frequency and modality with which cardiac imaging should be performed in patients during and after cancer therapy; their surveillance protocols are based on the methodology from clinical trials and expert opinions.15,61 The imaging modality (echocardiography, radionuclide ventriculography, and cardiac magnetic resonance imaging) for cardiac surveillance of LV function will depend on local access and expertise as well as the clinical scenario, with preference given to echocardiography with 3-dimensional imaging techniques due to its portability and easy access.15,61 In the case of anthracyclines, the international expert consensus recommends a baseline echocardiogram evaluation, with a follow-up at the completion of therapy, and 6 months later. If the dose is higher than 240mg/m2, an evaluation before each additional cycle is recommended.15 The European Society of Medical Oncology recommends echocardiograms at baseline, at the completion of therapy, every 3months within the first 12 months, then every year.61 With respect to VEGF-inhibitors, the international expert consensus recommends a baseline echocardiogram evaluation, with follow-up at 1 month and every 3 months during therapy.15 Regarding HER2-targeted therapies, however, there appears to be consensus to assess LV function at baseline and every 3 months during therapy.15,31,65
Long-term monitoring of cancer survivors depends mostly on the presence of modifier risk factors and the use of a strategy of early detection of subclinical LV dysfunction during therapy, accordingly to the international expert consensus.15 In the absence of concomitant CV risk or prior radiotherapy, if the global longitudinal strain is stable during chemotherapy and is normal 6 months after the completion of anthracycline-based therapy, or troponins have remained negative throughout therapy, additional imaging surveillance for CTRCD is not necessary. In patients who were not monitored with strain imaging or troponin, a yearly clinical CV assessment is suggested, searching for early signs and symptoms of CVDs. Patients who have received concomitant radiation need to be followed up according to published American Society of Echocardiography and European Association of Cardiovascular Imaging expert consensus.66 The National Comprehensive Cancer Network recommends echocardiography within 1 year of completion of cancer therapy. In the case of older cancer survivors an increased vigilance is recommended, particularly in instances of high-dose anthracycline exposure.61 Long-term imaging surveillance of children, adolescents, and young adult cancer survivors is based on age at time of treatment and cumulative anthracycline dose as well as concomitant radiotherapy.67
The value of surveillance in general is unclear.68,69 One reason is the lack of evidence that early detection strategies for LV dysfunction will improve the CV and overall outcomes of these patients.31 Thus, there is an urgent need for collaborative studies to guide patient management. Large prospective registries will enable the development of risk models for predicting CV events among cancer survivors as well as to evaluate the effect of surveillance strategies for cardiotoxicity prevention.31
CONCLUSIONIn this new era of cancer therapy, providers are more aware of the spectrum of CV toxicities that can occur during or after therapy and which can compromise the overall survival of the patient. Multidisciplinary cardio-oncology programs are being developed to improve patient care and outcomes. Collaboration is at the heart of this interdisciplinary field. Cardio-oncology is a rapidly evolving area of medicine and future work is under way to elucidate the most effective preventive interventions for the cardio-oncology patient.
CONFLICTS OF INTERESTNone declared.