Keywords
INTRODUCTION
In patients with acute myocardial infarction (AMI), restoration of patency in the infarct-related artery (IRA) is beneficial for survival.1,2 Fibrinolysis is effective therapy for this purpose. Nevertheless, the success rates of this treatment range from 75% to 90% according to the series and the type of fibrinolytic agent used.3 Patients who do not achieve reperfusion have a poorer prognosis than those in whom thrombolysis is successful.4
Rescue percutaneous transluminal coronary angioplasty (PTCA) refers to a strategy for mechanical reperfusion of the IRA in cases in which fibrinolysis has failed.5 Observational studies have shown that this technique is effective for improving the left ventricular ejection fraction (LVEF)6 and attaining TIMI III flow in the affected vesssel.5 The few existing randomized studies investigating survival in these patients have shown no improvement with the use of PTCA.7-9 However, these studies are not recent and they excluded high-risk cases, used only balloon PTCA, and did not take into account new interventional devices, such as systems for distal protection and thrombectomy. In addition, the indication for PTCA was not based on clinical criteria, but instead on angiographic criteria following elective post-thrombolysis coronary angiography. A more recent study has shown that reperfusion of the IRA is associated with increased long-term survival in patients with cardiogenic shock.10
The main objective of this study was to determine the factors predicting mortality at 30 days in patients undergoing rescue PTCA indicated on the basis of clinical criteria. The secondary aim was to determine the long-term evolution and establish the predictors of associated mortality in these patients.
PATIENTS AND METHODS
Selection of Patients
This prospective cohort study included 71 consecutive, unselected patients who came to the coronary unit of our hospital for rescue PTCA from January 2000 to August 2002. The cohort represented 20.5% of patients with AMI receiving fibrinolysis and 27% of patients with coronary syndrome and elevated ST segment undergoing PTCA.
The study included all patients treated with fibrinolysis for AMI of less than 24 hours' evolution with clinical criteria of reperfusion failure. The criteria to define reperfusion failure were the following: persistence of ST segment elevation >50% with respect to the admission electrocardiogram (ECG) at 90 min after the initiation of fibrinolysis, with or without chest pain, a new ST segment elevation with or without a new episode of pain, or the presence of cardiogenic shock. We excluded patients with AMI of more than 24 hours' evolution, those with cardiogenic shock secondary to a mechanical complication or with hypovolemia, patients in shock who did not receive fibrinolysis because of the time of evolution, and those who underwent elective post-thrombolysis PTCA in relation to a research protocol.
Procedure and Definitions
Before the procedure, 500 mg of aspirin was administered to all patients except those with contraindications. Angioplasty was performed with a standard technique, using femoral access in all cases. Stent implantation was attempted in all patients; abciximab was used according to the criteria of the attending interventionist at a bolus dose of 0.25 mg/kg, followed by 12 hours' perfusion at 0.25 µg/kg/min. During PTCA, heparin was administered at a weight-adjusted dose of 100 U/kg or 70 U/kg in patients receiving abciximab, in order to maintain an activated clotting time (ACT) between 200 and 250 s. All patients received a loading dose of 300 mg of clopidogrel, followed by 75 mg of clopidogrel every 24 h or 250 mg of ticlopidine every 12 h thereafter for 1 month.
Angioplasty was defined as successful when TIMI II or TIMI III flow was obtained in the IRA without complications during the procedure.11 Percentage of stenosis was estimated by visual inspection and IRA patency was determined at the first contrast injection using the classification established in the TIMI studies.12
Major bleeding complications were defined as any intracranial or retroperitoneal hemorrhage, any bleeding associated with a hemoglobin decrease above 5 g/dL, or bleeding requiring transfusion.
In all patients the enzyme profile was performed every four hours until creatine kinase (CK) peak had been reached, and electrocardiography was done during the first 7 days following infarction.
Data were recorded on the time between the onset of pain and initiation of fibrinolysis, and between the onset of pain and PTCA. Clinical, angiographic and procedure-related variables were analyzed. Clinical follow-up consisted of a medical visit at day 30 and a telephone contact at one year.
Statistical Analysis
The statistical analysis was performed with SPSS, version 11.2. Continuous variables are presented as mean ± standard deviation (SD) and categorical variables as percentage. For the mortality analysis, continuous variables were compared with Student's t test, and categorical variables with the Chi-square test or Fisher's exact text, where appropriate. A logistic regression model was used to identify predictors of mortality, introducing the significant variables (P<.05) found in the univariate analysis in the model, in order to preserve parsimony. The backward stepwise method was used for this purpose. A model was assigned to study associations among the independent variables. The survival curve was obtained with the Kaplan-Meier method. Significance was set at a two-tailed P-value of <.05.
RESULTS
Baseline Characteristics
The baseline characteristics of the 71 patients are shown in Table 1. There were 18 patients in cardiogenic shock (25.3%), among whom 11 cases (15.5%) were secondary to left ventricular dysfunction, and the remaining patients had right ventricular AMI.
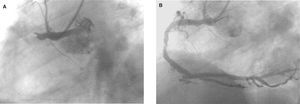
Overall, 33 (46.5%) patients presented TIMI 0-I flow before PTCA. An example of one patient with TIMI 0 flow is shown in Figure. The mean percentage of stenosis was 91%±11.6%. The IRA was identified and treated with stent implantation in all except 2 cases (97.2%); the remaining patients were treated with balloon angioplasty alone. A second stenosis located in an artery unrelated to the infarction was treated during the same procedure in two patients. The IRA was considered to be the left anterior descending (LAD) artery in 46.5% and the right coronary artery in 46.5%. Before PTCA, a visible thrombus was observed in 62% of the patients. Angiographic success was achieved in 69 (97.2%) patients, with TIMI II flow in 10 (14%) and TIMI III flow in 59 (83%). Direct stent implantation was performed in 27 (38%) patients. Abciximab was administered in 17 (26.5%) patients and balloon counterpulsation was used in 5 of the 11 (45.4%) patients with left ventricular cardiogenic shock.
Figure 1. A: example of proximal right coronary artery occlusion in a patient who had undergone prior fibrinolysis with tenecteplase. B: outcome following rescue angioplasty.
In-Hospital Course and Evolution at 30 Days
Among the 71 patients included, 7 (9.8%) had died at 30 days of follow-up. The causes of death were cardiogenic shock in 5 (71.4%) patients, intracranial bleeding in 1 (14.3%), and sudden death in 1 (14.3%). Six (8.5%) patients presented with clinical symptoms of left heart failure that resolved with medical treatment, and the remaining 58 (81.7%) had an uncomplicated course. One patient (1.4%) who presented with subacute stent occlusion 48 h after PTCA was treated with balloon angioplasty with good angiographic outcome. The patient showed no CK re-elevation or appearance of new Q waves on the ECG. Mortality due to cardiogenic shock secondary to left ventricular dysfunction was 45.4% in our series, whereas there were no deaths in patients with right ventricular dysfunction.
Major bleeding complications occurred in 4 patients (5.6%): 2 patients presented with intracranial hemorrhage and 2 required transfusion. None of these patients had received abciximab. Five (7%) patients presented with a moderate hematoma at the puncture site, but they did not require transfusion.
Analysis of Mortality
Univariate analysis showed that the patients who died were older (69.6±12 vs 60.3±10 years; P=.027), presented more frequently with Killip class III-IV (71.4% vs 21.9%; P=.013), and experienced a significantly smaller interval between onset of pain and PTCA (5.6±1.7 vs 9.1±5.0 h; P=.001) than those who survived. There was a significant association between higher mortality and ST segment elevation in more than 7 leads (37.5% vs 6.7%; P=.031) and a larger number of Q waves (5.8±1.5 vs 3.4±1.4; P<.001) on the ECG. In addition, post-AMI LVEF was significantly lower in these patients (30.0±7.1% vs 48.5±13.1%; P=.007). These factors and others exhibiting no association with mortality are shown in Table 2.
Among the angiographic and procedure-related variables (Table 3), LAD as the culprit artery (85.7% vs 42.2%; P=.04) and failure of PTCA (28.6% vs 0%; P=.008) were associated with higher mortality.
Multivariate analysis was performed to determine the independent predictors of mortality at 30 days (Table 4). The variables identified as predictors of mortality included age (odds ratio [OR]=1.2; 95% confidence interval [CI], 1.03-1.5; P=.001), Killip III-IV class (OR=20.1; 95% CI, 1.7-500; P=.003), PTCA failure (OR=indeterminate; P=.04), and LAD as the culprit artery (OR=12.6; 95% CI, 0.7-214.9; P =.04).
Long-Term Evolution
Cumulative mortality at 1 year of follow-up was 11.3% (clinical follow-up in 98.6% of patients). In the multivariate analysis the independent predictors of long-term mortality included age (OR=1.1; 95% CI, 1-1.2; P=.038), Killip class III-IV (OR=18.9; 95% CI, 2-166.6; P =.005), and LAD as the culprit artery (OR=12.6; 95% CI, 1.02-157.4; P =.02).
DISCUSSION
The results of this study suggest that rescue PTCA is an effective technique for achieving revascularization of the infarct-related artery, with a success rate of 97.2%, similar to that reported in other series13-15 and comparable to that obtained following primary PTCA.16,17 Moreover, our study suggests that rescue PTCA with stent implantation may be a safe technique with a low rate of subacute thrombosis (1.4%).
In the randomized studies published to date,7-9 rescue PTCA is indicated after IRA occlusion has been detected following a specific protocol. Clinical criteria are used for indicating rescue PTCA only in some descriptive studies.13-15,18 Among these, La Vecchia et al13 analyzed the long-term evolution of selected patients with extensive AMI (ST segment elevation in more than 4 leads, Killip class >1 or LVEF <40% by echocardiography) undergoing rescue PTCA. At 2 years, age and LVEF were identified as predictors of mortality.
In our series, we included all consecutive patients undergoing rescue PTCA, without taking into consideration the extent of the infarction or the patient's hemodynamic status (e.g., cardiogenic shock). Eighteen of the patients included were in cardiogenic shock, and in 8 of them the indication for PTCA was the presence of cardiogenic shock per se, since they had ECG criteria of reperfusion. The remaining patients in shock (10 patients) additionally presented ECG criteria of reperfusion failure. There were no significant differences with regard to delay of fibrinolysis (2.4±1.4 vs 2.0±1.3 h; P=.6) or pain-to-needle time (7.8±4 vs 9.1±5.1 h; P=.3) between cardiogenic shock patients with ECG criteria of reperfusion and the remaining patients. Thus, delays in IRA reperfusion can be excluded as the cause of shock in these patients. In those in whom the indication for PTCA was the presence of shock alone, the incidence of IRA occlusion was 25%. In contrast, 70% of patients with shock and no evidence of reperfusion had an occluded IRA, a higher incidence than that reported in other rescue PTCA studies based on clinical criteria.13,15 Therefore, in the absence of a mechanical complication, cardiogenic shock in a patients with fibrinolysis-treated AMI could be a sign of IRA reperfusion failure. This fact is highly relevant in the light of findings from the SHOCK10 study, which demonstrated higher survival in cardiogenic shock patients with successful IRA revascularization.
In the present study, 15% of the patients experienced cardiogenic shock due to left ventricular dysfunction. Mortality in these patients was 45%, whereas overall mortality was 9.8% at 30 days and 11.3% at 1 year. These data are similar to those obtained in the study by La Vecchia et al,13 in which 15% of the patients were in cardiogenic shock (in-hospital mortality 8.1% and long-term mortality 11.4%).
The following independent predictors of 30-day mortality were identified: age, Killip class III-IV, LAD as the IRA and PTCA failure. Age, LAD involvement, and Killip class are classic factors associated with mortality in AMI.19,20 In addition, PTCA failure has been associated with elevated mortality, reaching up to 50% in some series of rescue PTCA.21 However, these studies excluded patients with cardiogenic shock. In the present study PTCA failure occurred in 2 patients (2.8%) with cardiogenic shock, but mortality in these cases was 100%. This high mortality following PTCA failure is similar to the rates observed in the SHOCK study when revascularization of the IRA is not achieved.10
The ECG findings are among the most important factors to consider when using clinical criteria to assess IRA reperfusion in patients with AMI undergoing fibrinolysis. A >50% ST segment decrease in the lead formerly showing the highest elevation has a positive predictive value of up to 87% when associated with the presence of reperfusion arrhymithias,22 and a diagnostic accuracy of 86%,23,24 as well as a better prognosis.
Randomized studies published to date have not demonstrated a clear benefit of rescue PTCA.7,8 The RESCUE7 study included 151 patients with anterior AMI undergoing thrombolysis with angiographic confirmation of IRA occlusion in a diagnostic coronary arteriography performed 90 min after fibrinolysis. The primary endpoint of the study, improvement of resting LVEF at 25-30 days, was not achieved. However, with PTCA there was a decrease in mortality and severe heart failure at 30 days (6.4% vs 56.6%; P=.05) and better LVEF results on exercise. Another randomized study8 included 28 patients with IRA occlusion following fibrinolysis (angiographic inclusion criteria). Rescue PTCA, once again, showed no benefits as compared to conservative treatment with regard to survival. A subsequent metaanalysis25 of these 2 reports did not detect differences between the 2 strategies.
The inconclusive results of these studies7,8 may have several explanations: a) the limited number of patients analyzed; b) the exclusion of high-risk patients (cardiogenic shock, prior AMI); c) the absence of a clinical indication for PTCA--Moreover, the presence of TIMI III flow in the IRA alone does not always indicate the existence of myocardial perfusion. Thu s, patients in whom > 50% of the ST-segment elevation is not corrected have a poorer prognosis than those in whom it is corrected, even when TIMI III flow is obtained in the IRA.26-29 This fact could partially explain why TIMI 0-I flow was only found in 46.5% of the cases¯ and lastly; d) balloon PTCA alone was used in these studies.
As compared to fibrinolysis, primary PTCA reduces 30-day mortality and the incidence of reinfarction, and achieves a higher rate of IRA patency.16,30,31 Nevertheless, not all hospitals provide 24-hour availability of this technique. In addition to rescue PTCA, other therapeutic strategies can be used, such as out-of-hospital fibrinolysis and subsequent transfer to a hospital providing PTCA, direct transfer of a patient to a center with PTCA, and facilitated angioplasty.
These 3 approaches have been assessed in various randomized studies. Bonnefoy et al32 demonstrated that out-of-hospital fibrinolysis with subsequent transfer of the patient to a hospital with emergency PTCA capability is comparable to primary PTCA in terms of mortality, AMI and stroke. In the DANAMI-233 study, 2 reperfusion strategies were evaluated in hospitals without PTCA facilities. In-hospital fibrinolysis was compared with an accelerated alteplase regimen with direct transfer to a hospital where PTCA could be performed. No differences between the groups were found with respect to mortality or stroke, but there was a higher rate of recurrent infarction in the group who received fibrinolysis.
Lastly, facilitated PTCA is being assessed in multicenter studies (FINESSE). Recent results from the GRACIA234 study, which randomized primary PTCA versus facilitated PTCA, have demonstrated no differences with regard to the extent of the AMI or LVEF values at 6 weeks.
The main limitations of the present study are its observational nature and the fact that a relatively small number of patients are included. Because there is no control group, we cannot establish the advantages of rescue PTCA over conservative management. Moreover, selection bias is preset, since we do not know whether all the patients with an indication of fibrinolysis received this treatment or whether rescue PTCA was requested for all patients without evidence of reperfusion. Nonetheless, this is the first study that identifies the predictors of mortality following rescue PTCA indicated on the basis of clinical criteria in non-selected patients. Therefore the results adhere more to daily practice, in which mainly clinical features and ECG findings provide the basis for making therapeutic decisions in these patients.
CONCLUSIONS
In the present study, rescue PTCA was observed to be a useful technique for achieving revascularization of the affected artery (98.2%). Failure of PTCA together with other factors, such as advanced age, hemodynamic status, and LAD as the culprit artery were associated with higher 30-day mortality. Although rescue PTCA was not shown to improve survival in early randomized studies, the lack of clinical trials in the current era of interventional procedures and the potential for improving LVEF reported in observational studies favors the use of this technique in routine practice for patients with clinical criteria of thrombolysis failure.
Correspondence: Dr. M. Sabaté.
Servicio de Cardiología Intervencionista. Hospital Clínico San Carlos.
Prof. Martín Lagos, s/n. 28040 Madrid. España.
E-mail: msabate.hcsc@salud.madrid.org