The angiographic and clinical efficacy of polymer-free sirolimus-eluting stents vs polymer-based paclitaxel-eluting stents remain a matter of debate. We sought to investigate angiographic and clinical measures of efficacy of polymer-free sirolimus-eluting stents vs polymer-based paclitaxel-eluting stents.
MethodsPatient data from the randomized intracoronary stenting and angiographic restenosis-test equivalence between the 2 drug-eluting stents (ISAR-TEST) clinical trial and the LIPSIA Yukon clinical trial (randomized comparison of a polymer-free sirolimus-eluting stent vs a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus) were pooled. The angiographic (primary) endpoint was in-stent late lumen loss at 6 months to 9 months. The clinical (secondary) endpoints were death or myocardial infarction, cardiac death or myocardial infarction, target lesion revascularization, and myocardial infarction.
ResultsA total of 686 patients (polymer-free sirolimus-eluting stents, n=345 vs polymer-based paclitaxel-eluting stents, n=341) and 751 lesions (polymer-free sirolimus-eluting stents, n=383 vs polymer-based paclitaxel-eluting stents, n=368) were included in the study. Control angiography (606 lesions, 80.6%) showed comparable in-stent late lumen loss for polymer-free sirolimus-eluting stents vs polymer-based paclitaxel-eluting stents (0.53 [0.59] mm vs 0.46 [0.57] mm; P=.15). Median follow-up was 34.8 months. Polymer-free sirolimus-eluting stents and polymer-based paclitaxel-eluting stents were associated with comparable risk of death or myocardial infarction (relative risk=1.17; 95% confidence interval, 0.49-2.80; P=.71), cardiac death or myocardial infarction (relative risk=1.17; 95% confidence interval, 0.72-1.89; P=.50), target lesion revascularization (relative risk=0.98; 95% confidence interval, 0.65-1.47; P=.93), and myocardial infarction (relative risk=1.79; 95% confidence interval, 0.85-3.76; P=.12).
ConclusionsIn this pooled analysis, polymer-free sirolimus-eluting stents were comparable to polymer-based paclitaxel-eluting stents with respect to both angiographic and clinical efficacy.
Keywords
.
IntroductionDrug-eluting stents (DES) reduce the need for repeat revascularization as compared to bare-metal stents.1 However, subsequent reinterventions to the target vessel may still be required and the initial enthusiasm for DES has been somewhat attenuated by results from extended follow-up studies.2 The main components of DES are drugs, supportive metallic or inorganic scaffolds, and polymers.3 Permanent (nondegradable) polymers are used to bind eluted drugs (both limus and nonlimus) to the scaffold and allow progressive release, thereby prolonging the duration of the antirestenotic effect. However, permanent polymers remain after drug elution and are involved in the chronic inflammatory response at the site of stent implantation and in late adverse clinical events.4,5
Strategies to avoid these consequences of permanent polymers include the use of biodegradable polymers, bioabsorbable DES, and polymer-free DES. Unlike polymer-based DES, polymer-free DES uses a mechanically modified strut surface to bind drugs directly without recourse to a polymer.6 Several studies have investigated polymer-free stents which elute the immunosuppressive drug sirolimus.7 However, recent publications have questioned their angiographic and clinical efficacy as compared to polymer-based paclitaxel-eluting stents (PB-PES), especially in high-risk subgroups.8
We performed an updated, individual patient level, pooled analysis of the intracoronary stenting and angiographic restenosis-test equivalence between the 2 DES (ISAR-TEST) clinical trial and the LIPSIA Yukon clinical trial9,10 (randomized comparison of a polymer-free sirolimus-eluting stent [PF-SES] vs a PB-PES in patients with diabetes mellitus). The main objective of the present study was to investigate the performance of PF-SES vs PB-PES in relation to angiographic and clinical outcomes. In addition, the performance of PF-SES vs PB-PES was assessed in subgroups of certain interest.
MethodsPatient Population and Study ProtocolThe design of the ISAR-TEST and LIPSIA Yukon clinical trials as well as the characteristics of the patients enrolled in these trials have been described previously9,10 and are listed in detail in, Table 1, supplementary material. In brief, both trials were prospective, multicenter, controlled clinical trials in which patients were randomized to receive PF-SES (Yukon or Yukon Choice, Translumina GmbH; Hechingen, Germany) or PB-PES (Taxus Express 2 or Taxus Liberté, Boston Scientific; Natick, Massachusetts, United States). Enrollment was completed in 2006 for ISAR-TEST and in 2008 for LIPSIA Yukon. Patients were eligible if they were =18 years old, had stable or unstable angina or a positive stress test, and if percutaneous coronary intervention for de novo lesions (=50%) in a native coronary artery was indicated. In the LIPSIA Yukon trial, only patients with diabetes mellitus were enrolled. Both trials mandated the exclusive use of randomized stents in cases where multiple lesions were treated or multiple stents were required. The main exclusion criteria were recent myocardial infarction (MI) (=48h after symptom onset), a target lesion or significant (=50%) stenosis located in the left main trunk, and contraindications or known allergies to contrast medium, acetylsalicylic acid, heparin, thienopyridines, sirolimus, paclitaxel, or stainless steel. Severe disorders of hemostasis or platelet aggregation, pregnancy, other trial participation, and severe comorbidities (ie, malignancy) were also considered as exclusion criteria.
Randomization Process and InterventionBoth studies were approved by the ethics committee at each participating institution, and eligible patients gave their written informed consent. After confirmation of the enrollment criteria and wiring of the target lesion, patients were randomly assigned to the treatment groups. A computer-generated random sequence list and sealed opaque envelopes were used in the ISAR-TEST trial; an internet-based randomization system was used in the LIPSIA Yukon trial. Randomization was not stratified in either of the trials. Patients were assigned to receive either the PF-SES or PB-PES; detailed description and in-depth background of the PF-SES have been published elsewhere.6,11 The ISAR-TEST trial used a first-generation Yukon stent scaffold, while the LIPSIA Yukon trial used the second generation platform (Yukon Choice). Although there were trivial differences in terms of strut thickness (115µm for first generation vs 87µm for second generation), the total amount of drug used for the coating process remained the same (2% sirolimus solution). This concentration of sirolimus had the highest antirestenotic efficacy in a previous report.6 The 2 PB-PES platforms used in the 2 trials—Taxus Express 2 in the ISAR-TEST and Taxus Liberté in the LIPSIA Yukon trial—have been consistently demonstrated to be equivalent.12 For the purpose of these studies, stents were available at a diameter of 2.0mm to 3.5mm and a length of 8mm to 25mm for PF-SES; and a diameter of 2.25mm to 3.50mm and a length of 8mm to 32mm for PB-PES. Standard periprocedural therapy consisted of acetylsalicylic acid, and unfractionated heparin (100 IU/kg) plus glycoprotein IIb/IIIa inhibitors administration if clinically indicated. A loading dose of 600mg of clopidogrel was given prior to intervention. Other cardio-active drugs were administered at the discretion of the treating physician. After discharge, patients received dual antiplatelet therapy for a minimum of 6 months up to 12 months. Acetylsalicylic acid therapy was ongoing thereafter.
Follow-up and Data ManagementAll patients were asked to return for follow-up coronary angiography between 6 months and 9 months after randomization, or earlier if angina had developed. Experienced personnel unaware of the treatment allocation performed quantitative coronary analysis of baseline, postimplantation, and follow-up angiograms (see supplementary material for further details). Relevant clinical data were collected, verified against source documentation, and entered into a computer database. Adverse events were adjudicated by event committees blinded to patient randomization.9,10
For the current analysis, data of all patients included in the original publications were merged in a database specifically designed for the purpose of the study. Individual data were transferred without patient identifiers to the ISAR research center (Deutsches Herzzentrum; Munich, Germany). The final dataset was checked for completeness and consistency and compared with the results from original publications. Data were managed according to the intention-to-treat principle.
Definitions of EndpointsIn the original publications, both the ISAR-TEST and the LIPSIA Yukon trials reported sample-size calculations adequate to investigate the angiographic noninferiority of PF-SES as compared to PB-PES. For the purpose of the current analysis, the angiographic (primary) endpoint consisted of in-stent late lumen loss (LLL) at 6-month to 9-month invasive surveillance. Clinical (secondary) endpoints were: death or MI, cardiac death or MI, and target lesion revascularization and MI. Per-protocol definitions were adopted according to the longest available follow-up (Table 2, supplementary material). ISAR-TEST reported the incidence of definite (angiographically confirmed) stent thrombosis (ST), whilst LIPSIA Yukon reported both definite and probable ST. In order to allow for a higher degree of specificity,13 only definite ST was considered in this pooled analysis.14
Statistical AnalysisThe patient-level databases from both trials were combined. Pooling of data was deemed allowable because the inclusion and exclusion criteria and the definition of endpoints were comparable for both the trials. In addition, treatment arms in the original trials were perfectly balanced, according to random allocation to treatments.9,10
Categorical data are presented as counts and percentages and were compared using either the chi-square test or the Fisher-exact test, where expected cell values were <5. Continuous data are presented as mean (standard deviation) and were compared using either the Student t test where data were normally distributed or the Wilcoxon rank sum test.
The Mantel-Cox method was used to stratify the trial and assisted in the analysis of clinical endpoints. Results were pooled according to the DerSimonian and Laird method for random effects.15 Treatment effect could not be assessed for trials in which the event of interest was not observed in any of the treatment groups. For trials in which only 1 of the treatment groups had no events of interest, the treatment effect estimate and its standard error were approximated from 2×2 contingency tables, after adding 0.5 to each cell. Data for patients who did not experience the event of interest were censored at the time of the last follow-up visit. Relative risk (RR) and its 95% confidence interval (95%CI) was used as a summary statistic. Heterogeneity across the studies was tested using the I2 statistic. This test describes the percentage of total variation across a trial that arises due to heterogeneity rather than chance. As a guide, I2 values<25% indicated low, I2 values between 25% to 50% indicated moderate, and I2 values>50% indicated high heterogeneity.16 Due to the small number of trials included, publication bias was not considered. An exploratory analysis evaluated angiographic and clinical endpoints within subgroups of interest. Subgroups were expressed as dichotomous variables. In every subgroup the first variable served as a reference for calculations. Specifically, the degree of LLL, the RR and its 95%CI of death or MI, cardiac death or MI, target lesion revascularization, and MI were evaluated according to sex (male/female), age (under/above the median value), diabetes (yes/no), treatment with insulin (yes/no), unstable presentation (yes/no), and vessel size (under/above the median value). In addition, the possible interaction between the treatment effect (receiving a PF-SES vs PB-PES) and the membership (or not) to each subgroup was explored using a linear regression model for in-stent LLL and the Mantel-Cox method for other endpoints. All analyses were on intention-to-treat basis.
For patients included in the ISAR-TEST trial who had multiple lesions, only the first treated lesion was included in the analysis. Although this method may introduce potential bias, it was applied to reduce the interlesion dependence of the risk for restenosis in patients receiving multilesion coronary stent placement.17 In the LIPSIA Yukon trial, a slight modification of intention-to-treat analysis was adopted for angiographic data: only the patients receiving at least 1 study stent in the target lesion were effectively included in the analysis.
This pooled analysis of the ISAR-TEST and LIPSIA Yukon trials was post hoc. In addition, since stratification to account for subgroups was absent at the time of randomization for either trial, the results of subgroup analysis remain hypothesis-generating in nature.
All tests were 2 tailed and a P<.05 indicated significance. Statistical analyses were performed with Stata 10.0 statistical software (STATA Corp.; College Station, Texas, United States).
ResultsIn the ISAR-TEST trial, with at 2 participating centers, 225 randomized patients received PF-SES and 225 patients received PB-PES.9 In the LIPSIA Yukon trial,10 3 participating centers randomized 120 patients to PF-SES and 120 patients to PB-PES; 4 patients were unavailable for analyses (2 patients withdrew consent; 2 patients were randomized twice). Therefore, for the present study, 686 (99.4%) of the 690 patients originally randomized were included in the analysis (PF-SES, n=345 vs PB-PES, n=341; figure, supplementary material). The total number of treated lesions was 751 (PF-SES, n=383 vs PB-PES, n=368).
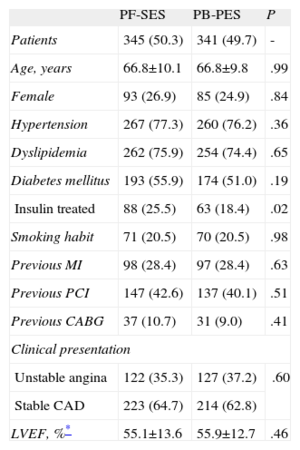
Baseline clinical characteristics were well matched between groups (Table 1). A high prevalence of diabetes was reported in the pooled population (55.9% for PF-SES vs 51.0% for PB-PES; P=.19) and the proportion of patients treated with insulin was higher for patients randomized to PF-SES than to PB-PES (25.5% vs 18.4%; P=.02).
Pooled Analysis of ISAR-TEST and LIPSIA Yukon Trials-Clinical Characteristics
| PF-SES | PB-PES | P | |
| Patients | 345 (50.3) | 341 (49.7) | - |
| Age, years | 66.8±10.1 | 66.8±9.8 | .99 |
| Female | 93 (26.9) | 85 (24.9) | .84 |
| Hypertension | 267 (77.3) | 260 (76.2) | .36 |
| Dyslipidemia | 262 (75.9) | 254 (74.4) | .65 |
| Diabetes mellitus | 193 (55.9) | 174 (51.0) | .19 |
| Insulin treated | 88 (25.5) | 63 (18.4) | .02 |
| Smoking habit | 71 (20.5) | 70 (20.5) | .98 |
| Previous MI | 98 (28.4) | 97 (28.4) | .63 |
| Previous PCI | 147 (42.6) | 137 (40.1) | .51 |
| Previous CABG | 37 (10.7) | 31 (9.0) | .41 |
| Clinical presentation | |||
| Unstable angina | 122 (35.3) | 127 (37.2) | .60 |
| Stable CAD | 223 (64.7) | 214 (62.8) | |
| LVEF, %* | 55.1±13.6 | 55.9±12.7 | .46 |
CABG, coronary artery by-pass graft; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PB-PES, polymer-based paclitaxel-eluting stents; PCI, percutaneous coronary intervention; PF-SES, polymer-free sirolimus-eluting stents.
Data are expressed as no. (%) or mean±standard deviation. A P-value of <.05 is considered significant.
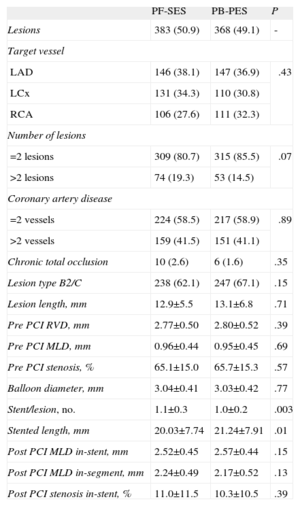
Angiographic data were adequately paired in the treatment arms (Table 2) with only minor differences in terms of stent/lesion ratio (1.1 [0.3] vs 1.0 [0.2]; P=.003) and mean stented length (20.03 [7.74] mm vs 21.24 [7.91] mm; P=.01) for PF-SES vs PB-PES, respectively. These discrepancies were due to differences in the dimensions of the stents available for the studies and were considered unlikely to be of any clinical significance. It is to be noted that a large proportion of complex lesions (B2/C) were treated (62.1% for PF-SES vs 67.1% for PB-PES; P=.15).
Pooled Analysis of ISAR-TEST and LIPSIA Yukon Trials-Angiographic and Procedural Characteristics
| PF-SES | PB-PES | P | |
| Lesions | 383 (50.9) | 368 (49.1) | - |
| Target vessel | |||
| LAD | 146 (38.1) | 147 (36.9) | .43 |
| LCx | 131 (34.3) | 110 (30.8) | |
| RCA | 106 (27.6) | 111 (32.3) | |
| Number of lesions | |||
| =2 lesions | 309 (80.7) | 315 (85.5) | .07 |
| >2 lesions | 74 (19.3) | 53 (14.5) | |
| Coronary artery disease | |||
| =2 vessels | 224 (58.5) | 217 (58.9) | .89 |
| >2 vessels | 159 (41.5) | 151 (41.1) | |
| Chronic total occlusion | 10 (2.6) | 6 (1.6) | .35 |
| Lesion type B2/C | 238 (62.1) | 247 (67.1) | .15 |
| Lesion length, mm | 12.9±5.5 | 13.1±6.8 | .71 |
| Pre PCI RVD, mm | 2.77±0.50 | 2.80±0.52 | .39 |
| Pre PCI MLD, mm | 0.96±0.44 | 0.95±0.45 | .69 |
| Pre PCI stenosis, % | 65.1±15.0 | 65.7±15.3 | .57 |
| Balloon diameter, mm | 3.04±0.41 | 3.03±0.42 | .77 |
| Stent/lesion, no. | 1.1±0.3 | 1.0±0.2 | .003 |
| Stented length, mm | 20.03±7.74 | 21.24±7.91 | .01 |
| Post PCI MLD in-stent, mm | 2.52±0.45 | 2.57±0.44 | .15 |
| Post PCI MLD in-segment, mm | 2.24±0.49 | 2.17±0.52 | .13 |
| Post PCI stenosis in-stent, % | 11.0±11.5 | 10.3±10.5 | .39 |
LAD, left anterior descending coronary artery; LCx, left circumflex artery; MLD, minimum lumen diameter; PB-PES, polymer-based paclitaxel-eluting stents; PCI, percutaneous coronary intervention; PF-SES, polymer-free sirolimus-eluting stents; RCA, right coronary artery; RVD, reference vessel diameter.
Data are expressed as no. (%) or mean±standard deviation. A P-value of <.05 is considered significant.
Of 686 patients undergoing PF-SES or PB-PES implantation, 552 patients (80.4%) returned for follow-up angiography. Of those who did not return, 24 patients had died (13 patients [19.4%] in the PF-SES group, and 11 patients [16.4%] in the PB-PES group; P=.94). Thus, 606 lesions (80.6%) were measured.
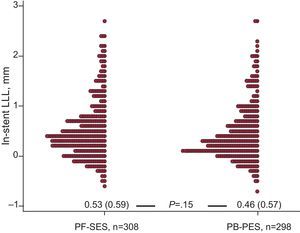
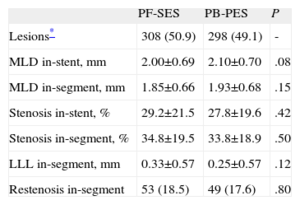
Regarding in-stent LLL, there was no significant difference between PF-SES and PB-PES (0.53 [0.59] mm vs 0.46 [0.57] mm; P=.15) (Fig. 1). Other components of angiographic surveillance are listed in Table 3. Interestingly, in-segment binary restenosis was similar for PF-SES and PB-PES (18.5% vs 17.6%; P=.80).
In-stent late lumen loss distribution (dot plot) at follow-up angiography in the 2 study groups. LLL, late lumen loss; PF-SES, polymer-free sirolimus-eluting stents; PB-PES, polymer-based paclitaxel-eluting stents. Cumulative data are presented as mean (standard deviation) and compared with the Wilcoxon rank sum test.
Pooled Analysis of ISAR-TEST and LIPSIA Yukon Trials-Other Components of Angiographic Surveillance
| PF-SES | PB-PES | P | |
| Lesions* | 308 (50.9) | 298 (49.1) | - |
| MLD in-stent, mm | 2.00±0.69 | 2.10±0.70 | .08 |
| MLD in-segment, mm | 1.85±0.66 | 1.93±0.68 | .15 |
| Stenosis in-stent, % | 29.2±21.5 | 27.8±19.6 | .42 |
| Stenosis in-segment, % | 34.8±19.5 | 33.8±18.9 | .50 |
| LLL in-segment, mm | 0.33±0.57 | 0.25±0.57 | .12 |
| Restenosis in-segment | 53 (18.5) | 49 (17.6) | .80 |
LLL, late lumen loss; MLD, minimum lumen diameter; PB-PES, polymer-based paclitaxel-eluting stents; PF-SES, polymer-free sirolimus-eluting stents.
Data are expressed as no. (%) or mean±standard deviation. A P-value of <.05 is considered significant.
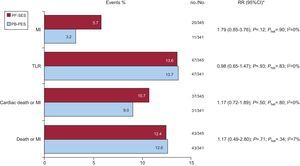
Clinical follow-up was completed in all patients (median 34.8 months). No significant difference in outcome was reported for PF-SES vs PB-PES with respect to death or MI (12.4% vs 12.6%; RR=1.17; 95%CI, 0.49-2.80]; P=.71), cardiac death or MI (10.7% vs 9.0%; RR=1.17; 95%CI, 0.72-1.89]; P=.50), target lesion revascularization (13.6% vs 13.7%; RR=0.98; 95%CI, 0.65-1.47]; P=.93), and MI (5.7% vs 3.2%; RR=1.79; 95%CI, 0.85-3.76]; P=.12). There was no significant heterogeneity across studies (Fig. 2). Definite ST occurred in 4 patients in the ISAR-TEST trial (0.2% [1] for PF-SES vs 0.8% [3] for PB-PES; P=.37). No definite ST was reported among patients enrolled in the LIPSIA Yukon trial.
Adverse events in the 2 study groups at clinical follow-up. 95%CI, 95% confidence interval; MI, myocardial infarction; no., events; No., total patients; PB-PES, polymer-based paclitaxel-eluting stents; PF-SES, polymer-free sirolimus-eluting stents; Phet, P for heterogeneity; RR, relative risk; TLR, target lesion revascularization. Data are presented as percentages (bar graphs), no./No., and Mantel-Cox (DerSimonian and Laird method for random effects) based RR (95%CI). * I2 statistics and Phet values are provided.
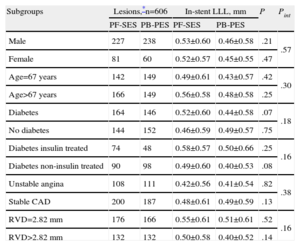
For exploratory purposes, linear regression and Mantel-Cox methods were used to assess whether in-stent LLL and clinical outcomes associated with PF-SES vs PB-PES were consistent within different subgroups (Tables 4 and 5). It is to be noted that, for PF-SES, the highest degree of in-stent LLL was reported in patients with insulin-treated diabetes (0.58 [0.57] mm); for PB-PES, the highest degree of in-stent LLL occurred in those with small vessel disease (0.51 [0.61] mm). There was no interaction between treatment effect (PF-SES vs PB-PES) and membership in any of the subgroups of interest.
Pooled Analysis of ISAR-TEST and LIPSIA Yukon Trials-Angiographic Endpoint According to Subgroup Analysis
| Subgroups | Lesions,*n=606 | In-stent LLL, mm | P | Pint | ||
| PF-SES | PB-PES | PF-SES | PB-PES | |||
| Male | 227 | 238 | 0.53±0.60 | 0.46±0.58 | .21 | .57 |
| Female | 81 | 60 | 0.52±0.57 | 0.45±0.55 | .47 | |
| Age=67 years | 142 | 149 | 0.49±0.61 | 0.43±0.57 | .42 | .30 |
| Age>67 years | 166 | 149 | 0.56±0.58 | 0.48±0.58 | .25 | |
| Diabetes | 164 | 146 | 0.52±0.60 | 0.44±0.58 | .07 | .18 |
| No diabetes | 144 | 152 | 0.46±0.59 | 0.49±0.57 | .75 | |
| Diabetes insulin treated | 74 | 48 | 0.58±0.57 | 0.50±0.66 | .25 | .16 |
| Diabetes non-insulin treated | 90 | 98 | 0.49±0.60 | 0.40±0.53 | .08 | |
| Unstable angina | 108 | 111 | 0.42±0.56 | 0.41±0.54 | .82 | .38 |
| Stable CAD | 200 | 187 | 0.48±0.61 | 0.49±0.59 | .13 | |
| RVD=2.82 mm | 176 | 166 | 0.55±0.61 | 0.51±0.61 | .52 | .16 |
| RVD>2.82 mm | 132 | 132 | 0.50±0.58 | 0.40±0.52 | .14 | |
CAD, coronary artery disease; LLL, late lumen loss; PB-PES, polymer-based paclitaxel-eluting stents; PF-SES, polymer-free sirolimus-eluting stents; Pint, P for interaction (linear regression model with interaction term); RVD, reference vessel diameter.
For age and RVD subgroups, median values were used to define cut-offs. A P-value of <.05 is considered significant.
Data are expressed as no. or mean±standard deviation.
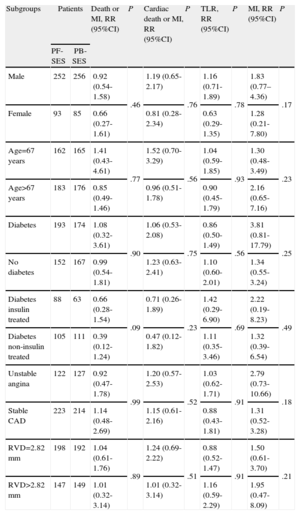
Pooled Analysis of ISAR-TEST and LIPSIA Yukon Trials-Relative Risk for Death or Myocardial Infarction, Cardiac Death or Myocardial Infarction, Target Lesion Revascularization, and Myocardial Infarction in Different Subgroups
| Subgroups | Patients | Death or MI, RR (95%CI) | P | Cardiac death or MI, RR (95%CI) | P | TLR, RR (95%CI) | P | MI, RR (95%CI) | P | |
| PF-SES | PB-SES | |||||||||
| Male | 252 | 256 | 0.92 (0.54-1.58) | .46 | 1.19 (0.65-2.17) | .76 | 1.16 (0.71-1.89) | .78 | 1.83 (0.77–4.36) | .17 |
| Female | 93 | 85 | 0.66 (0.27-1.61) | 0.81 (0.28-2.34) | 0.63 (0.29-1.35) | 1.28 (0.21-7.80) | ||||
| Age=67 years | 162 | 165 | 1.41 (0.43-4.61) | .77 | 1.52 (0.70-3.29) | .56 | 1.04 (0.59-1.85) | .93 | 1.30 (0.48-3.49) | .23 |
| Age>67 years | 183 | 176 | 0.85 (0.49-1.46) | 0.96 (0.51-1.78) | 0.90 (0.45-1.79) | 2.16 (0.65-7.16) | ||||
| Diabetes | 193 | 174 | 1.08 (0.32-3.61) | .90 | 1.06 (0.53-2.08) | .75 | 0.86 (0.50-1.49) | .56 | 3.81 (0.81-17.79) | .25 |
| No diabetes | 152 | 167 | 0.99 (0.54-1.81) | 1.23 (0.63-2.41) | 1.10 (0.60-2.01) | 1.34 (0.55-3.24) | ||||
| Diabetes insulin treated | 88 | 63 | 0.66 (0.28-1.54) | .09 | 0.71 (0.26-1.89) | .23 | 1.42 (0.29-6.90) | .69 | 2.22 (0.19-8.23) | .49 |
| Diabetes non-insulin treated | 105 | 111 | 0.39 (0.12-1.24) | 0.47 (0.12-1.82) | 1.11 (0.35-3.46) | 1.32 (0.39-6.54) | ||||
| Unstable angina | 122 | 127 | 0.92 (0.47-1.78) | .99 | 1.20 (0.57-2.53) | .52 | 1.03 (0.62-1.71) | .91 | 2.79 (0.73-10.66) | .18 |
| Stable CAD | 223 | 214 | 1.14 (0.48-2.69) | 1.15 (0.61-2.16) | 0.88 (0.43-1.81) | 1.31 (0.52-3.28) | ||||
| RVD=2.82 mm | 198 | 192 | 1.04 (0.61-1.76) | .89 | 1.24 (0.69-2.22) | .51 | 0.88 (0.52-1.47) | .91 | 1.50 (0.61-3.70) | .21 |
| RVD>2.82 mm | 147 | 149 | 1.01 (0.32-3.14) | 1.01 (0.32-3.14) | 1.16 (0.59-2.29) | 1.95 (0.47-8.09) | ||||
95%CI, 95% confidence interval; CAD, coronary artery disease; MI, myocardial infarction; PB-PES, polymer-based paclitaxel-eluting stents; PF-SES, polymer-free sirolimus-eluting stents; RR, relative risk; RVD, reference vessel diameter; TLR, target lesion revascularization.
RR and 95%CI were used as summary statistics; P-values for interaction between treatment effects (PF-SES vs PB-PES) and subgroups were derived using a Mantel-Cox model. For age and RVD subgroups, median values were used to define cut-offs. Subgroups are expressed as dichotomous variables. In every subgroup the first variable is the reference for calculations. A P-value of <.05 is considered significant.
This is the first patient-level pooled analysis of 2 randomized, controlled, multicenter trials investigating coronary revascularization with PF-SES vs PB-PES. The main findings are: a) treatment with PF-SES as compared with PB-PES led to a comparable degree of LLL at 6-month to 9-month control angiography; b) treatment with PF-SES as compared to PB-PES led to comparable risk of clinical events at long-term follow-up; c) hypo-thesis-generating subgroup analysis did not show treatment-effect modification for angiographic and clinical endpoints within subgroups (sex, age, diabetes, insulin treatment, clinical presentation, and vessel size), and d) no interaction between treatment effect and membership to any of the subgroups was found.
Animal studies have documented ongoing vessel wall inflammation for >12 months after permanent-polymer DES implantation.18 This inflammation is considered to be responsible for a wide spectrum of clinical syndromes including systemic hypersensitivity reactions,19 late malapposition, and late ST.20 In addition, this chronic process might be responsible for neointimal proliferation and the late “catch-up” phenomenon associated with polymer-based DES.21 Both polymer-free DES and biodegradable-polymer DES have arisen as alternative strategies to polymer-based DES: once the drug elution is completed, the absence of polymer over stent struts might militate against the development of an adverse reaction to the stent.5
The ISAR-TEST trial documented a noninferior angiographic and clinical efficacy of PF-SES vs PB-PES at 9-month follow-up,9 and more recently a similar efficacy and safety at 5-year follow-up.22 These results are in line with previous findings suggesting that antirestenotic efficacy for PF-SES is sustained compared to polymer-based DES, irrespective of eluted drug (sirolimus or paclitaxel).23 The 2-year angiographic data from the ISAR-TEST 224 and the ISAR-TEST 325 trials showed persistent, significant, progressive renarrowing of vessels treated with polymer-based stents. Restenosis associated with polymer-free platforms did not progress between 6 months and 2 years. However, conflicting observations have cast doubt on the efficacy of polymer-free platforms, with the performance of PF-SES in diabetics regarded as a case in point. Diabetes has the potential to erode the antiproliferative advantage of limus-eluting stents over PB-PES.26,27 In the LIPSIA Yukon trial, which enrolled only subjects with diabetes, PF-SES was inferior to PB-PES in terms of angiographic performance, without clinical consequences.10 On the contrary, a recent randomized trial reported a superior antirestenotic efficacy of a polymer-free sirolimus-eluting platform vs PB-PES.28 Most importantly, this superiority was also confirmed in patients with diabetes.
The present study included the largest population randomly treated with PF-SES vs PB-PES to date. At angiographic surveillance and a clinical follow-up of almost 3 years, both stent platforms showed a similar antirestenotic efficacy. As compared to the original trials,9,10 here we considered a longer follow-up in a larger population at a higher risk of restenosis, which is pivotal to challenging the antirestenotic efficacy of different stent platforms.29 In addition, notwithstanding their exploratory nature, subgroup analyses aimed to further investigate the angiographic and clinical performance of PF-SES vs PB-PES in very challenging subsets of patients, such as those who are older or have diabetes, unstable presentation, or small vessel disease.
The angiographic findings of the present study require careful consideration. The relatively high proportion of complex lesions and high-risk patients may have led to a higher degree of LLL as compared to other studies.28,30 Specifically, in patients receiving PF-SES, the degree of in-stent LLL observed among diabetics and especially in those requiring insulin may raise potential concern. On the one hand, it is a feature of insulin analogues to reduce the antiproliferative efficacy of limus drugs.26 On the other hand, it remains to be defined whether the lack of significant differences between PF-SES and PB-PES in the present analysis was due to the sample size or to the use of PB-PES as a (weak) comparator. However, recent trials found superior or comparable efficacy of polymer-free stent platforms, with highly-lipophilic sirolimus formulations, in comparison with PB-PES28 and second-generation DES,31 respectively.
A similar risk of adverse clinical events was observed after PF-SES and PB-PES implantation. Importantly, the reported risk of target lesion revascularization reflected findings from previous studies, with comparable follow-up.25 In this regard, although the time course of neointimal suppression is dynamic and varies among DES by virtue of the polymer-dependent drug-release kinetics, the interactions between factors affecting long-term stent performance may not be resolved at the time of short-term angiographic follow-up. This might explain why different degrees of LLL with PF-SES vs PB-PES did not translate into clinical differences at long-term follow-up.23–25
For the purpose of this study, only definite ST was considered. In accordance with previous reports, this pooled analysis found a very low incidence of definite ST among patients allocated in the PF-SES and PB-PES arms.23 Although the present study was not powered to investigate the occurrence of such a rare event and the hypothesis that polymer-free DES may have lower thrombogenicity remains unproven,32 the observed low rate of ST may be somewhat reassuring.
In the present study, main outcomes were evaluated for the study population as a whole and within selected key subgroups. Although there was no evidence of treatment-effect modification within any of the subgroups of interest, this analysis was exploratory in nature and based on an insufficient sample size to allow for definite conclusions. Further investigation is needed to shed more light on any differential efficacy between PF-SES and PB-PES in specific subgroups.
Study LimitationsThis pooled analysis presents some limitations. First, the ISAR-TEST and LIPSIA Yukon trials evaluated the noninferiority of angiographic efficacy of PF-SES vs PB-PES. Thus, all of the current results must be regarded as post hoc and hypothesis-generating. In addition, the present study was underpowered to adequately assess relatively infrequent adverse events such as death, MI, and ST. Second, PF-SES was compared with PB-PES in this study. PB-PES is among the most often implanted first-generation devices and considered as an adequate comparator at the time of inception of the ISAR-TEST and LIPSIA Yukon trials. However, PB-PES is no longer available in most countries and this may limit the clinical relevance of current analyses, although recent trials still used PB-PES as a control arm.28,30 Moreover, investigations of PB-PES at long-term follow-up and in high-risk subgroups of patients (ie, patients with diabetes) remain of some interest because in these subsets the efficacy of limus-eluting stents represents a matter of controversy.26,27 Third, we did not perform long-term angiographic follow-up that could be useful in determining the time-course of endothelial regrowth and the relationship between angiographic and clinical outcomes. Moreover, it must be acknowledged that 6-month to 9-month angiographic surveillance data are based on incomplete observations from only 80.4% of the total patient cohort. Notwithstanding, the LLL data appear consistent with target lesion revascularization data, which were available for the entire cohort. Fourth, very long-term follow-up would have been of certain interest to shed more light on late events associated with the polymer coatings. Finally, although the population enrolled might be perceived as high-risk, it resulted from 2 randomized trials for which somewhat stringent inclusion/exclusion criteria had been applied.
ConclusionsThis pooled analysis suggests that PF-SES with respect to PB-PES may lead to a comparable degree of LLL at angiographic surveillance, as well as a similar risk of death, target lesion revascularization, and MI.
Conflicts of interestProfessor Kastrati and professor Schömig hold a patent for a microporous stent surface. Dr. Desch and Professor Thiele received an unrestricted grant from Translumina GmbH (Hechingen, Germany). The remaining authors declare no potential conflicts of interest.