The advent of stents in interventional cardiology has contributed to excellent initial angiographic outcomes in all lesions, avoiding acute or subacute vessel occlusion and significantly reducing angiographic and clinical restenosis. Perhaps paradoxically, stents have been found to induce greater neointimal proliferation than balloon angioplasty. In fact, the best results are based on the virtual elimination of early elastic recoil and dissection (which guarantees an excellent immediate result) and avoidance of late remodeling of the coronary wall. Increased neointimal growth resulting from injury to the vascular wall by the metal stent is therefore “accommodated” and a larger coronary lumen is guaranteed in the long-term. The drug-eluting stents (DES) introduced more than a decade ago have inspired a new revolution in our specialty, drastically reducing neointimal hyperplasia and the need for reintervention.1 The unprecedented results obtained with DES have allowed us to offer percutaneous revascularization to patients with increasingly unfavorable clinical and anatomic characteristics.1 Some patients treated with DES do experience restenosis, but this problem is rare. The initial enthusiasm generated by DES has, however, been somewhat dampened by confirmation that these devices do not reduce the risk of stent thrombosis. In fact, they simply delay onset, whereby the risk of very late thrombosis (after the first year) could be higher than that with conventional stents.2 While this complication occurs only in exceptional circumstances, it has significant clinical implications. The international scientific community responded unanimously to this issue, initially with recommendations of concern and caution, and subsequently by the promotion of new research efforts. The optimization and duration of antiplatelet therapy were critically reviewed and the development of new DES was stimulated.2
Stent thrombosis is a multifactorial phenomenon.2–5 It is known that mechanical problems in the metal stent platform (underexpansion, incomplete apposition, deformation, or even rupture)3 and adverse drug reactions (initially only sirolimus [immunosuppresor] or paclitaxel [anti-proliferative]), manifested by toxicity in the vascular wall, excessive remodeling, delay or lack of endothelialization, and endothelial dysfunction,5 could be implicated in the pathogenesis of this complication (Figure). However, the long-term direct contact between the polymer and the arterial wall has caused special concern, as experimental and clinical evidence point toward this contact as a cause of late vascular toxicity with chronic inflammation, fibrin deposits, and even local allergic reactions in some patients.5 More recent findings show that “neoatherosclerosis” (more frequent and earlier with DES implantation) can occasionally form the nexus between restenosis and very late stent thrombosis,3 although clinical recognition is not yet common. All these issues favor the creation of a new adverse anatomical category, that we have called the “vulnerable stent”,2 which sustains the risk of thrombosis over an extended period.
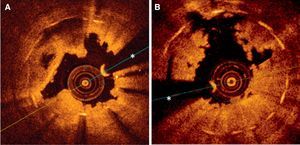
Optical coherence images obtained following initial thrombus aspiration in patients with thrombosis after implantation of drug-eluting stents. A: Subacute thrombosis associated with early interruption of dual antiplatelet therapy; the shadow of the thrombus obstructs the view of some metallic elements; in other areas, this stent showed clear underexpansion and struts not yet endothelialized. B: Very late thrombosis; the irregular shape of the residual thrombus is seen, leaving very little shadow and areas with incomplete apposition (at 11 hours); in other areas of this stent, underexpansion is seen and some metallic elements are not covered. *Artifact produced by angioplasty guidewire.
DES basically consist of a metal platform, the polymer, and the drug.6 The polymer provides a stable medium that contains the drug to be administered and releases it into the arterial wall at a predetermined rate. Managing this particular step has been one of the most difficult challenges in the development of DES. Similarly, continued efforts to improve antiproliferative capacity and, above all, to avoid “vulnerable stents” have led to advances in knowledge and new technological developments. Fortunately, many significant improvements have been made to all DES components in recent years6: second-generation DES have new platforms for advanced alloys (cobalt-chromium, platinum-chromium) that provide greater radial strength and much thinner, more flexible structures6; we have more advanced and potent lipophilic drugs (everolimus, zotarolimus, biolimus) at our disposal; and the new permanent polymers have improved greatly, and not only are used in lower quantities (ultrafine) but are more “friendly” to the vascular wall (biocompatible polymers).6
Recent data from randomized clinical trials (selected populations) and from broad general activity registers (real-world population) indicate that some of the new second-generation DES provide greater safety and efficacy than their first-generation counterparts.6 Some sources indicate that the risk of thrombosis with second-generation DES may even be lower than with conventional metal stents.8 In certain settings, the feeling of “renewed confidence” is so widespread that reductions have been suggested to the duration of combined antiplatelet therapy. In fact, the efficacy obtained with first-generation DES was already excellent (with sirolimus DES outperforming paclitaxel DES), making it extremely difficult to demonstrate any clinically significant improvements.7 Better safety is also hard to prove, given that evidence of any significant reduction in rare complications such as late stent thrombosis would require studies tracking thousands of patients over long periods of time. Consequently, the approach to this issue has necessarily been pragmatic and new clinical trials with DES have usually been designed to determine noninferiority rather than superiority. As discussed below, another approach to this problem is to perform meta-analyses of new studies. Finally, surrogate endpoints (intracoronary analysis of neointimal growth or of endothelialization) can be used to highlight the superiority of the new DES. Although such studies are of undoubted interest, both from a mechanical and pathophysiological point of view, their results obviously lack the same practical implications as those of superiority trials with primary clinical end points.
Alongside the intensive work on second-generation DES, another strategy has focused on avoiding the problems arising from the permanent contact between the polymer and the arterial wall. Some researchers have suggested this strategy will lead to a new (third) generation of DES, although their classification continues to cause controversy. In turn, this strategy has 2 clearly differentiated alternatives. The first option is to incorporate the drug directly into the metal stent platform (with no polymer), using new technology9–11 to overcome the limitations previously encountered in some initial prototypes of polymer-free stents (mainly their lack of effectiveness). Microporous metal platforms are currently available that allow the desired concentrations of the drug to be retained on the surface,9,11 while other platforms offer sophisticated designs with microreservoirs to hold the pharmaceutical product.10 Some very interesting results have been produced with new polymer-free DES that incorporate the drug directly into the platform,9–11 but many of these are still considered to be at the preliminary or proof-of-concept stage.
The second option, which has created enormous interest, is the development of biodegradable polymers.12 These new polymers fulfill their initial function of predetermined drug release before completely disappearing from the arterial wall, leaving behind a conventional metal stent that will support the vascular architecture and help prevent late negative remodeling. One of the most significant challenges for biodegradable polymers is to synchronize degradation and the predetermined end of drug release. Many of the new biodegradable polymers are based on polylactic acid compounds. This material is hydrolyzed and degrades to lactic acid, which is finally metabolized via the Krebs cycle to generate CO2 and H2O. Moreover, a number of these new DES are extremely versatile; some allow several drugs with synergic or complementary effects to be administered simultaneously and others have biodegradable polymers that release the antiproliferative drug exclusively through the abluminal surface (in contact with the wall); their adluminal surface is coated with antibodies that capture endothelial progenitor cells. The aim here is to combine efficacy strategies for antirestenosis with safety elements to stimulate endothelialization. Interesting data are currently appearing on biodegradable polymer DES. From the mechanical perspective, optical coherence tomography studies have demonstrated that endothelialization occurs earlier with these devices than with first-generation DES. More importantly, recent clinical evidence from long-term follow-up data on these devices (4 years) showed a lower incidence of very late thrombosis compared with first-generation permanent-polymer DES.12
A final alternative is the fully bioabsorbable implantable devices that are available for clinical use.13 In these systems, both the polymer and the entire platform eventually disappear. Some metals, such as magnesium are fully degraded by biocorrosion, but the most advanced platforms are once again based on polylactic acid structures. These devices have the incomparable theoretical attractiveness of allowing complete restoration of function to the vascular wall on dissolution, enabling both acute vasomotor changes and more progressive vascular remodeling. They also avoid the “corset effect” and eliminate the later mechanical problems associated with badly aligned or protruding platforms, or those blocking access to lateral branches. No less significant is the fact that they facilitate noninvasive visualization and evaluation during monitoring of the treated coronary segments. The results of some observational studies in selected patients with favorable lesions have been, quite simply, spectacular.13 The most recent structural designs have yielded late angiographic findings that rival those obtained with second-generation DES. Finally, intracoronary diagnostic techniques have confirmed the complete disappearance of the device after 2 years.13 Although the current profile of these systems and their mechanical (plastic) characteristics could limit widespread clinical use, several studies are currently looking into outcomes and possible indications in various clinical and anatomical contexts.
A NEW META-ANALYSISIn Revista Española de Cardiología, Cassese et al.14 present an interesting meta-analysis of 2 randomized studies comparing polymer-free and permanent polymer DES. The polymer-free DES has a microporous metallic platform, loaded with 2% sirolimus using a special device housed in the hospital's cath lab. The data from the 2 randomized studies were pooled to analyze all of the individual patient data in a way that undoubtedly increases the value of the meta-analysis. Although one of the studies included all types of patients while the other concentrated exclusively on diabetic individuals, a test for heterogeneity confirmed the validity of the joint analysis. This meta-analysis not only provides the broadest evidence of the outcomes of polymer-free DES, but also incorporates additional clinical tracking of all the patients in a way that provides deeper insight into their long-term clinical progress.14 The 2 original randomized studies had a noninferiority design that included a relatively limited number of patients and provided only short-term clinical tracking. The new meta-analysis included 686 patients (with 751 treated lesions) followed-up for 3 years (100% clinical tracking). The patients generally had complex angiographic and clinical characteristics (53% patients with diabetes, 2 or 3 type B2-C lesions). In this meta-analysis, both the late angiographic outcomes and the patients’ final clinical outcomes were similar with both types of DES. In addition, the results concured across all of the different subgroups of interest in a way that supports the consistency and robustness of the findings. More relevant still is the fact that there was just a single episode of definitive thrombosis amongst the 345 patients treated with polymer-free DES throughout the tracking period.14 While these results are of great interest, they also prompt a number of reflections. First, the permanent-polymer DES used for comparison was a first-generation paclitaxel DES. As the authors acknowledge, this DES is not currently considered ideal for purposes of comparison because its late angiographic outcomes are inferior to those of first-generation sirolimus DES. Second, in-stent late loss of 0.53mm is very high for a DES containing a –limus-type drug, even though the result was obtained in an population group with unfavorable characteristics (diabetes and complex lesions). The authors do, however, provide adequate discussion of the possible implications of drug type in patients with diabetes. Also, although the angiographic differences were not statistically significant in terms of outcomes for the paclitaxel DES (late loss of 0.46mm; P=.15), the confidence margins were relatively broad; therefore, the results may have been different in an analysis of a larger number of patients. It should be remembered that a previous study comparing the same DES reported worse results for the polymer-free DES.15 However, this particular study was observational and retrospective, and the authors acknowledge that, due to its greater flexibility, the polymer-free DES could have been used preferentially in patients with more complex lesions. Other previous studies have also questioned the relative value of polymer-free DES with both paclitaxel16 and sirolimus.17 However, the meta-analysis suggests that polymer-free DES have at least the same efficacy as first-generation polymer DES and provides very interesting data on their long-term safety. Furthermore, previous studies by the same researchers indicate that the presence of polymer can result in significant very late lumen loss (post-9 months, in a late catch-up effect), which does not appear to occur with polymer-free DES.9 By the same token, optical coherence studies indicate a greater endothelialization of polymer-free DES than of polymer DES. Finally, the most recent data indicate that the efficacy of new-generation polymer-free DES incorporating new drugs (amphilimus or sirolimus/probucol) is greater than that of first-generation DES10 and is similar to that of second-generation DES.11 All this clearly illustrates that this line of research is both open and highly active, but also indicates that further studies are needed to analyze the outcomes of the new generations of polymer-free DES and to confirm the potential advantages of this treatment strategy.
FINAL CONSIDERATIONSSome researchers criticize the efforts of interventional cardiologists, always proudly comparing their results with new stents. They berate this type of research (comparison of stent A with stent B) as overly simplistic, stating that it reflects a lack of creativity and, above all, that it is intrinsically linked to the interests of the industry. We cannot share that opinion. Percutaneous intervention is currently the most widely used strategy for coronary revascularization.1 It is our responsibility to offer our patients the best available treatment at any given time. Only rigorous and critical comparisons, undertaken in a clinical context with analysis of efficacy and effectiveness outcomes involving a sufficient number of patients followed-up over the necessary period of time will permit us to determine the true contributions of new devices. In interventional cardiology, we have learned from repeated disappointments that some devices are incredibly attractive from the pathophysiology point of view but can prove incapable of obtaining the desired clinical outcomes. In fact, this type of study represents the much-needed final stage of an exciting translational research exercise that will allow the consolidation of biotechnological innovation for the benefit of our patients. The Munich group behind the meta-analysis published in Revista Española de Cardiología14 offers a true example of coherence and perseverance in this spirit. Over the past decade, these researchers have performed an infinite number of randomized studies (ISAR studies) of the highest quality, allowing us to advance our understanding of the clinical usefulness of the new DES.
We can currently administer drugs directly to the arterial wall in a simple and efficacious manner, using a range of very different technologies. Drug-eluting balloons (avoiding the need for stent implantation) are very effective in the treatment of stent restenosis, but their recent use in de novo lesions is also the object of intense study.18 As we have indicated, new generations of biocompatible-polymer DES,8 biodegradable-polymer DES,12 and also fully bioabsorbable devices13 are now available. Finally, we have completely polymer-free DES capable of efficacious local drug administration.9–11 All these treatment strategies are in continual development, but at the same time must compete with each other in order for us to identify those that will eventually be confirmed as the “dominant treatment” for any given group of patients on the basis of their clinical and anatomic characteristics. The meta-analysis by Cassese et al.14 provides a new, fascinating, and dynamic piece of the puzzle, truly representative of the continuing advances enjoyed by those of us in the field of coronary interventionism.
CONFLICTS OF INTERESTNone declared.
.