The cusp overlap technique (COT) has been proposed to reduce conduction disturbances (CD) after transcatheter aortic valve implantation (TAVI) with self-expanding supra-annular devices, but there are scarce data on COT with intra-annular valves. The aim of this study was to determine whether the use of the COT during Portico implantation results in higher valve implantation and lower rates of CD.
MethodsWe included 85 patients undergoing TAVI with the Portico FlexNav system: 43 retrospective patients using the standard 3-cusp view and 42 prospective patients with the COT. Primary endpoints were implantation depth and new-onset CD (composite outcome of new-onset left bundle branch block and new permanent pacemaker implantation).
ResultsCOT resulted in a higher implantation depth (noncoronary cusp: 4.9±3.9 vs 7.4±3.0; P=.005) and lower new-onset CD (31.0% vs 58.1%; P=.012), with a tendency toward a lower need for permanent pacemaker implantation (14.3% vs 30.2%, P=.078; 7.7% vs 31.0%; P=.011 in patients without pre-existing right bundle branch block). Transvalvular aortic gradients were slightly lower with COT (8.7±3.7 vs 11.0±6.1; P=.044). There were no differences in technical success or major procedure-related complications. On multivariate analysis, COT use was associated with a lower risk of new-onset CD.
ConclusionsUse of the COT during Portico implantation is feasible and facilitates a higher valve implant, which in turn may help to reduce rates of new-onset CD.
Keywords
During the last decade, transcatheter aortic valve implantation (TAVI) has become the first-choice therapy for treating symptomatic severe aortic stenosis in most patients older than 80 years with suitable transfemoral access.1 However, the most common complication following TAVI remain new-onset conduction disturbances (CD).2 In recent years, several studies have shown the usefulness of a novel, modified implantation technique using a cusp overlap technique (COT), which overlaps the left and right coronary cusps and isolates the noncoronary cusp.3,4 The potential benefits of using the COT during TAVI include elongation of the left ventricular outflow tract, which provides more accurate control of the real prosthesis implantation depth, thus lowering the risk of interaction with the conduction system. Whereas early experiences of this approach using self-expanding supra-annular valves have been promising, to date, no studies have assessed the use of the COT using the self-expanding intra-annular Portico FlexNav system (Abbott Vascular, United States). The present study sought to determine whether the use of the COT during Portico FlexNav system implantation results in higher implantation depth and lower CD rates.
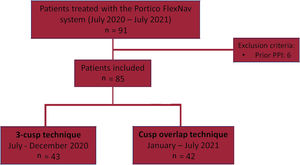
METHODSThis was a multicenter study including consecutive patients undergoing transfemoral TAVI with the self-expanding intra-annular Portico valve with the second-generation FlexNav delivery system at 3 tertiary centers. Between July 2020 and July 2021, a total of 91 consecutive patients underwent TAVI with the Portico FlexNav system. Patients with previous permanent pacemaker implantation (PPI) were excluded (n=6), leading to a final study population of 85 patients. Of these, 42 prospective patients received a Portico valve using the COT (January-July 2021) and were compared with 43 consecutive patients who had previously received a Portico valve using the traditional coplanar 3-cusp technique (July-December 2020) (figure 1). All implantations were performed by certified operators with a previous cumulative experience of at least 25 procedures with the first-generation Portico delivery system and ≥ 5 procedures with the next-generation FlexNav delivery system. Data were collected in accordance with the ethics committee of each participating center, and all patients provided signed informed consent to undergo the procedures.
Portico FlexNav systemThe Portico TAVI system is a self-expanding, intra-annular, repositionable transcatheter heart valve for the treatment of severe aortic stenosis showing favorable hemodynamic performance.5 The second-generation FlexNav delivery system includes a hydrophilic coated, integrated sheath (14- or 15-Fr equivalent) and a stability layer to minimize manipulations and facilitate a more gradual and controlled deployment of the valve, The system and received the CE mark approval in March 2020.6
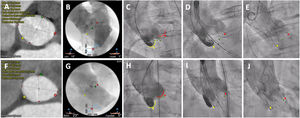
Implantation techniquePreprocedural work-up for anatomic assessment and optimal implant projections were made with either 3Mensio Structural Heart (Pie Medical Imaging, Netherlands) or Heart Navigator (Philips Healthcare, Netherlands). Predilation with a balloon diameter equal to the average diameter of the aortic annulus was recommended. Patients in the conventional (coplanar 3-cusp) deployment technique underwent valve implantation as previously described.5 Implantation using the COT was performed as follows: the valve was deployed in a right anterior oblique/caudal view, using no rapid pacing or controlled pacing at 120 bpm at the discretion of the implanting physician, followed by a left anterior oblique projection (modified coplanar view) to measure the left coronary depth and position of the delivery catheter before final release (figure 2). Target implantation depth was defined as ranging between 3 and 5mm (distance from the noncoronary cusp to the inflow of the transcatheter heart valve frame) regardless of the working projection. In both groups, the final implantation depth was assessed in a left anterior oblique angiogram.
Standard 3-cusp view (LAO) vs cusp overlap (RAO/CAU) technique. A-E: conventional 3-cusp implantation technique. A-C: cardiac computed tomography and baseline aortogram showing the classic coplanar projection with 3 cusps: NCC (yellow) on the left side, RCC (green) in the middle and LCC (red) on the right side. D-E: initial positioning of a Portico valve and final assessment under a 3-cusp view. F-J: cusp overlap implantation technique. F-H: computed tomography and angiogram in a RCC/LCC cusp overlap view: in this projection, the NCC (yellow) is on the left side and the RCC (green) and LCC (red) are overlapped on the right side. I-J: valve positioning and final assessment in a cusp overlap view. Dotted lines represent implantation depth (distance from the NCC to the ventricular end of the TAVI frame). CAU, caudal; LAO, left anterior oblique; LCC, left coronary cusp; NCC, noncoronary cusp; RAO, right anterior oblique; RCC, right coronary cusp.
Primary endpoints were prosthesis implantation depth and new-onset CD. Implant depth was assessed during final angiography after valve deployment removing device parallax and measuring the distance from the noncoronary and left coronary cusps to the deepest portion of the transcatheter heart valve. New-onset CD was defined as a composite outcome of new-onset left bundle branch block and new PPI. The decision for PPI was made in accordance with the 2019 consensus pathway.2 Technical success and in-hospital complications were defined according to the Valve Academic Research Consortium 3.7
Statistical analysisCategorical variables are expressed as number (percentage) and continuous variables as mean±standard deviation (SD) or median [interquartile range (IQR)]. Group comparisons were analyzed using the Student t test or its nonparametric equivalent, the Mann-Whitney U test for continuous variables and the chi-square test or Fischer exact test for categorical variables. Multivariate analysis through logistic regression was used to evaluate independent predictors of new-onset CD in the global population. Variables with P ≤ .10 on univariate analysis and those considered clinically relevant (eg, right bundle branch block) were entered into a multivariable logistic regression; less than 1 variable for every 10 events was included to avoid overfitting. A 2-sided P <.05 was considered significant for all statistical tests. Statistical analyses were performed with STATA version 14.0 (StataCorp LP, College Station, United States).
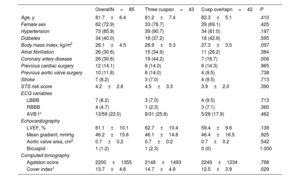
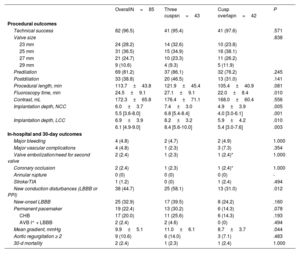
RESULTSStudy populationBetween July 2020 and July 2021, a total of 85 consecutive patients underwent TAVI with the Portico FlexNav system. The standard 3-cusp view was used in 43 patients, while in 42 patients TAVI were implanted using the COT. The main baseline and TAVI procedure characteristics of the study population are shown in table 1. The mean age was 82±6 years, with 73% of women, and a mean STS-PROM score of 4.2±2.8%. Pre-existing left or right bundle branch block was present in 13% of the patients. The mean gradient was 46.2±15.6mmHg, with an average Agatston calcium score on computed tomography of 2200±1355 AU. There were no significant differences between groups, except for a lower rate of coronary artery disease in the COT group (17% vs 44%; P=.006).
Baseline clinical characteristics
| OverallN=85 | Three cuspsn=43 | Cusp overlapn=42 | P | |
|---|---|---|---|---|
| Age, y | 81.7±6.4 | 81.2±7.4 | 82.3±5.1 | .410 |
| Female sex | 62 (72.9) | 33 (76.7) | 29 (69.1) | .425 |
| Hypertension | 73 (85.9) | 39 (90.7) | 34 (81.0) | .197 |
| Diabetes | 34 (40.0) | 16 (37.2) | 18 (42.9) | .595 |
| Body mass index, kg/m2 | 28.1±4.5 | 28.9±5.3 | 27.3±3.5 | .097 |
| Atrial fibrillation | 26 (30.6) | 15 (34.9) | 11 (26.2) | .384 |
| Coronary artery disease | 26 (30.6) | 19 (44.2) | 7 (16.7) | .006 |
| Previous cardiac surgery | 12 (14.1) | 6 (14.0) | 6 (14.3) | .965 |
| Previous aortic valve surgery | 10 (11.8) | 6 (14.0) | 4 (9.5) | .738 |
| Stroke | 7 (8.2) | 3 (7.0) | 4 (9.5) | .713 |
| STS risk score | 4.2±2.8 | 4.5±3.3 | 3.9±2.0 | .390 |
| ECG variables | ||||
| LBBB | 7 (8.2) | 3 (7.0) | 4 (9.5) | .713 |
| RBBB | 4 (4.7) | 1 (2.3) | 3 (7.1) | .360 |
| AVB I° | 13/59 (22.0) | 8/31 (25.8) | 5/28 (17.9) | .462 |
| Echocardiography | ||||
| LVEF, % | 61.1±10.1 | 62.7±10.4 | 59.4±9.6 | .139 |
| Mean gradient, mmHg | 46.2±15.6 | 46.1±14.8 | 46.4±16.5 | .925 |
| Aortic valve area, cm2 | 0.7±0.2 | 0.7±0.2 | 0.7±0.2 | .542 |
| Bicuspid | 1 (1.2) | 1 (2.3) | 0 (0) | 1.000 |
| Computed tomography | ||||
| Agatston score | 2200±1355 | 2148±1493 | 2249±1234 | .768 |
| Cover index* | 13.7±4.6 | 14.7±4.9 | 12.5±3.9 | .029 |
AVB I°, first degree atrioventricular block in patients with sinus rhythm; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block; STS, Society of Thoracic Surgeons.
The values are expressed as mean±standard deviation or No. (%).
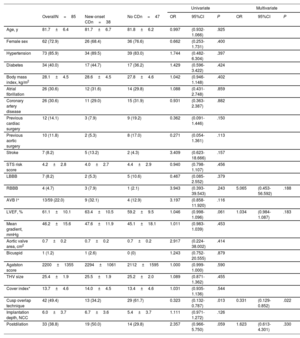
Procedural and 30-day data are depicted in table 2. Technical success was achieved in 97% of the patients, with no differences between groups. Predilation was used in 69 (81%) patients and postdilation was required in 33 (39%). Overall, there were no differences in major procedure-related complications. Two patients required a second valve due to device migration (1 in each group, 2.4%), one of them resulting in coronary occlusion successfully resolved by valve snaring and implantation of a second valve. There were 2 deaths at 30 days (2.4%), 1 in each group.
Procedural and clinical outcomes
| OverallN=85 | Three cuspsn=43 | Cusp overlapn=42 | P | |
|---|---|---|---|---|
| Procedural outcomes | ||||
| Technical success | 82 (96.5) | 41 (95.4) | 41 (97.6) | .571 |
| Valve size | .838 | |||
| 23 mm | 24 (28.2) | 14 (32.6) | 10 (23.8) | |
| 25 mm | 31 (36.5) | 15 (34.9) | 16 (38.1) | |
| 27 mm | 21 (24.7) | 10 (23.3) | 11 (26.2) | |
| 29 mm | 9 (10.6) | 4 (9.3) | 5 (11.9) | |
| Predilation | 69 (81.2) | 37 (86.1) | 32 (76.2) | .245 |
| Postdilation | 33 (38.8) | 20 (46.5) | 13 (31.0) | .141 |
| Procedural length, min | 113.7±43.8 | 121.9±45.4 | 105.4±40.9 | .081 |
| Fluoroscopy time, min | 24.5±9.1 | 27.1±9.1 | 22.0±8.4 | .010 |
| Contrast, mL | 172.3±65.8 | 176.4±71.1 | 168.0±60.4 | .556 |
| Implantation depth, NCC | 6.0±3.7 | 7.4±3.0 | 4.9±3.9 | .005 |
| 5.5 [3.6-8.0] | 6.8 [5.4-8.4] | 4.0 [3.0-6.1] | .001 | |
| Implantation depth, LCC | 6.9±3.9 | 8.2±3.2 | 5.9±4.2 | .010 |
| 6.1 [4.9-9.0] | 8.4 [5.6-10.0] | 5.4 [3.0-7.6] | .003 | |
| In-hospital and 30-day outcomes | ||||
| Major bleeding | 4 (4.8) | 2 (4.7) | 2 (4.9) | 1.000 |
| Major vascular complications | 4 (4.8) | 1 (2.3) | 3 (7.3) | .354 |
| Valve embolization/need for second valve | 2 (2.4) | 1 (2.3) | 1 (2.4)* | 1.000 |
| Coronary occlusion | 2 (2.4) | 1 (2.3) | 1 (2.4)* | 1.000 |
| Annular rupture | 0 (0) | 0 (0) | 0 (0) | - |
| Stroke/TIA | 1 (1.2) | 0 (0) | 1 (2.4) | .494 |
| New conduction disturbances (LBBB or PPI) | 38 (44.7) | 25 (58.1) | 13 (31.0) | .012 |
| New-onset LBBB | 25 (32.9) | 17 (39.5) | 8 (24.2) | .160 |
| Permanent pacemaker | 19 (22.4) | 13 (30.2) | 6 (14.3) | .078 |
| CHB | 17 (20.0) | 11 (25.6) | 6 (14.3) | .193 |
| AVB I° + LBBB | 2 (2.4) | 2 (4.6) | 0 (0) | .494 |
| Mean gradient, mmHg | 9.9±5.1 | 11.0±6.1 | 8.7±3.7 | .044 |
| Aortic regurgitation ≥ 2 | 9 (10.6) | 6 (14.0) | 3 (7.1) | .483 |
| 30-d mortality | 2 (2.4) | 1 (2.3) | 1 (2.4) | 1.000 |
AVB I°, first degree atrioventricular block; CHB, complete heart block; LBBB, left bundle branch block; LCC, left coronary cusp; NCC, noncoronary cusp; PPI, permanent pacemaker implantation; TIA, transient ischemic attack.
The values are expressed as mean±standard deviation, median [IQR] or No. (%).
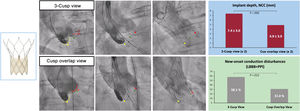
Use of the COT was associated with higher prosthesis implantation depth (noncoronary cusp: 4.9±3.9 vs 7.4±3.0; P=.005; left coronary cusp: 5.9±4.2 vs 8.2±3.2; P=.010) and lower rates of new-onset CD (31.0% vs 58.1%; P=.012), with a tendency toward a lesser need for permanent pacemaker implantation (14.3% vs 30.2%; P=.078; 7.7% vs 31.0%; P=.011 in patients without pre-existing right bundle branch block) (figure 3). All pacemakers were implanted during the index TAVI admission and pacemaker requirement was more likely in patients with pre-existing right bundle branch block (3/4: 75%), all in the COT group.
Central illustration. Use of the cusp overlap view resulted in higher implantation depth and lower rates of new-onset conduction disturbances compared with TAVI using the standard 3-cusp coplanar view. LBBB, left bundle branch block; NCC, noncoronary cusp; PPI, permanent pacemaker implantation.
Patients in the COT group exhibited lower transvalvular aortic gradients (8.7±3.7 vs 11.0±6.1; P=.044) and lower fluoroscopy time (22.0±8.4 vs 27.1±9.1, P=.010), with no differences in residual aortic regurgitation.
Predictors of new-onset conduction disturbancesThe main predictors of new-onset CD are summarized in table 3. On multivariable analysis, use of the COT was independently associated with a decreased risk of CD post-TAVI (odds ratio, 0.331, 95% confidence interval [95%CI], 0.129-0.852 P=.022).
Predictors of new-onset conduction disturbances after TAVI in global population
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OverallN=85 | New-onset CDn=38 | No CDn=47 | OR | 95%CI | P | OR | 95%CI | P | |
| Age, y | 81.7±6.4 | 81.7±6.7 | 81.8±6.2 | 0.997 | (0.932-1.066) | .925 | |||
| Female sex | 62 (72.9) | 26 (68.4) | 36 (76.6) | 0.662 | (0.253-1.731) | .400 | |||
| Hypertension | 73 (85.9) | 34 (89.5) | 39 (83.0) | 1.744 | (0.482-6.304) | .397 | |||
| Diabetes | 34 (40.0) | 17 (44.7) | 17 (36.2) | 1.429 | (0.596-3.422) | .424 | |||
| Body mass index, kg/m2 | 28.1±4.5 | 28.6±4.5 | 27.8±4.6 | 1.042 | (0.946-1.148) | .402 | |||
| Atrial fibrillation | 26 (30.6) | 12 (31.6) | 14 (29.8) | 1.088 | (0.431-2.748) | .859 | |||
| Coronary artery disease | 26 (30.6) | 11 (29.0) | 15 (31.9) | 0.931 | (0.363-2.387) | .882 | |||
| Previous cardiac surgery | 12 (14.1) | 3 (7.9) | 9 (19.2) | 0.362 | (0.091-1.446) | .150 | |||
| Previous aortic surgery | 10 (11.8) | 2 (5.3) | 8 (17.0) | 0.271 | (0.054-1.361) | .113 | |||
| Stroke | 7 (8.2) | 5 (13.2) | 2 (4.3) | 3.409 | (0.623-18.666) | .157 | |||
| STS risk score | 4.2±2.8 | 4.0±2.7 | 4.4±2.9 | 0.940 | (0.798-1.107) | .456 | |||
| LBBB | 7 (8.2) | 2 (5.3) | 5 (10.6) | 0.467 | (0.085-2.552) | .379 | |||
| RBBB | 4 (4.7) | 3 (7.9) | 1 (2.1) | 3.943 | (0.393-39.543) | .243 | 5.065 | (0.453-56.592) | .188 |
| AVB I° | 13/59 (22.0) | 9 (32.1) | 4 (12.9) | 3.197 | (0.858-11.920) | .116 | |||
| LVEF, % | 61.1±10.1 | 63.4±10.5 | 59.2±9.5 | 1.046 | (0.998-1.096) | .061 | 1.034 | (0.984-1.087) | .183 |
| Mean gradient, mmHg | 46.2±15.6 | 47.6±11.9 | 45.1±18.1 | 1.011 | (0.983-1.039) | .453 | |||
| Aortic valve area, cm2 | 0.7±0.2 | 0.7±0.2 | 0.7±0.2 | 2.917 | (0.224-38.002) | .414 | |||
| Bicuspid | 1 (1.2) | 1 (2.6) | 0 (0) | 1.243 | (0.752-20.555) | .879 | |||
| Agatston score | 2200±1355 | 2294±1061 | 2112±1595 | 1.000 | (0.999-1.000) | .590 | |||
| THV size | 25.4±1.9 | 25.5±1.9 | 25.2±2.0 | 1.089 | (0.871-1.362) | .455 | |||
| Cover index* | 13.7±4.6 | 14.0±4.5 | 13.4±4.6 | 1.031 | (0.935-1.136) | .544 | |||
| Cusp overlap technique | 42 (49.4) | 13 (34.2) | 29 (61.7) | 0.323 | (0.132-0.787) | .013 | 0.331 | (0.129-0.852) | .022 |
| Implantation depth, NCC | 6.0±3.7 | 6.7±3.6 | 5.4±3.7 | 1.111 | (0.971-1.272) | .126 | |||
| Postdilation | 33 (38.8) | 19 (50.0) | 14 (29.8) | 2.357 | (0.966-5.750) | .059 | 1.623 | (0.613-4.301) | .330 |
AVB I°, first degree atrioventricular block in patients with sinus rhythm CD, conduction disturbances; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NCC, noncoronary cusp; RBBB, right bundle branch block; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve replacement; THV, transcatheter heart valve.
Values are expressed as mean±standard deviation or No. (%).
The present study specifically assessed the safety and efficacy of the COT during TAVI with the self-expanding intra-annular Portico FlexNav system. The main findings of the study can be summarized as follows: a) use of the COT was feasible, with high technical success and low rates of major complications, similar to those achieved with the conventional coplanar 3-cusp view, b) COT use resulted in a higher implantation depth, and c) COT use was independently associated with lower rates of new-onset CD.
The COT has been proposed for deployment of self-expanding valves to enable higher device implantation and reduce postprocedural CD. The rationale behind this fluoroscopic projection, which isolates the most inferior hinge point of the noncoronary cusp in a right anterior oblique/caudal view, relies on the fact that annular contact with self-expanding valves occurs mainly from the noncoronary cusp toward the left coronary cusp.8 The potential advantages of this approach are elimination of the delivery system parallax, elongation of the left ventricular outflow tract and shorter visual distance of the aortic annulus (minor axis), enabling a more precise assessment of the real implantation depth, with the potential to minimize the risk of injury to the conduction system.9 The majority of experiences reported so far with this approach have been performed with the supra-annular Evolut valve (Medtronic, United States). In a propensity score analysis of 444 patients treated with Evolut, use of the COT (compared with the classic implantation technique) reduced the rate of PPI (11.8% vs 21.7%; P=.03) without compromising TAVI outcomes.10 In the largest series of consecutive patients undergoing Evolut TAVI using COT (n=694), Gada et al.11 demonstrated a very low risk of PPI (< 5%) with this technique, with a low rate of major cardiac adverse events.
To date, there have been scarce data on the COT with the Portico valve. In a retrospective study analyzing the impact of the COT with different self-expanding valves, Mendiz et al.12 included 19 patients treated with Portico. Overall and in line with prior studies, COT reduced the rate of CD without compromising safety outcomes.
Our results also support the use of the COT with the Portico FlexNav system as a safe strategy, with comparable success rates and similar rates of major complications. Indeed, using this approach resulted in higher valve implantation and subsequent reduction in the rates of conduction abnormalities after TAVI, without increased risk of valve embolization or compromise of valve hemodynamics. Interestingly, incorporation of this modified working projection may reduce radiation exposure (lower fluoroscopy time), by providing a more precise visualization of the implant depth and accurate device placement, although it did not translate into a reduction of the overall length of the procedure.
In the present work, COT was barely used in valve-in-valve procedures (figure 1 of the supplementary data). Albeit less frequent, this method can also be used to treat degenerated surgical valves, since surgical prostheses are directly aligned with native aortic valve commissures and consequently the postsurgical anatomy usually matches the native anatomy. Akin to TAVI in native valves, the cusp overlap view can be obtained by isolating the bioprosthetic stent post between the left and right coronary cusps based on preprocedural computed tomography, as recently reported by Wong et al.13
Of note, residual transvalvular gradients were lower in the COT group, which may be explained by a higher positioning of the leaflets leading to more favorable hemodynamics, although this finding should be interpreted as hypothesis-generating only.
Importantly, patients in the COT group had significantly lower rates of conduction abnormalities, similarly to previous reports with other self-expanding valves. The need for PPI in the standard 3-cusp group was roughly twice that in the COT group (30% vs 14%; P=.078) although this difference was not statistically significant probably due to the limited sample size. The difference was much more noticeable when we excluded patients with pre-existing right bundle branch block (31.0% vs 7.7%; P=.011), who are at the highest risk of PPI regardless of the type of valve and implantation technique.
Finally, postdilation was associated with increased new-onset CD post-TAVI on univariate but not on multivariate analysis. Whereas valvuloplasty may increase the risk of conduction abnormalities after TAVI due to the mechanical trauma to the conduction system, no clear association has yet been identified between postdilation and new PPI.14
LimitationsThis study has the limitations inherent to an observational retrospective study without an external adjudication event committee and a limited sample size. Implant depth was assessed by experienced, but not blinded operators, and could therefore be subject to bias. Postprocedural cardiac computed tomography was not systematically performed, so implantation depth was mainly assessed by angiography and the degree of neocommissural alignment could not be analyzed. Finally, because of the time frame of the study (and to minimize the factors that may influence the accuracy of valve placement at the desired annular position other than the implantation technique), it included only patients who received the Portico valve with the FlexNav delivery system, rather than the newer-generation Navitor valve that became commercially available in Spain in July 2021, which may limit generalizability of the findings. The newest best practice recommendations for Navitor valve implantation have incorporated the COT and clinical outcomes with the new device will be evaluated in the near future.
CONCLUSIONSApplication of the COT during implantation of the Portico FlexNav system is feasible and may help to achieve higher implantation depth and lower subsequent rates of new-onset CD without compromising safety outcomes or valve performance.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSL. Asmarats, L. Nombela-Franco, A. Regueiro and D. Arzamendi conceived and designed the analysis. L. Gutierrez-Alonso, G. Tirado-Conte, P. Cepas and E. Fernández-Peregrina collected the data. L. Asmarats and X. Millán performed the analysis. CH. Li, P. Jiménez-Quevedo and X. Freixa reviewed and edited the manuscript.
CONFLICTS OF INTERESTL. Asmarats, L. Nombela-Franco, X. Millán, CH. Li, X. Freixa and D. Arzamendi are proctors for Abbott. A. Regueiro is proctor for Abbott and Meril Life. The other authors report no conflicts.
- •
Conduction disturbances remain the most common complication of TAVI.
- •
The COT has been proposed to reduce conduction disturbances after TAVI with self-expanding supra-annular valves (mainly Evolut). However, scarce data exist on the safety and potential benefits of this new implant strategy with intra-annular devices.
- •
This study specifically assessed the safety and efficacy of the COT during TAVI with the self-expanding intra-annular Portico FlexNav system.
- •
Use of the COT during Portico implantation is feasible and facilitates a higher valve implant, which in turn may help to reduce the rates of new-onset CD.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.02.003