.
INTRODUCTIONThe choice of vascular coronary conduits depends on several factors, including the patient's intrinsic characteristics (age, body mass index, diabetes, pulmonary function, peripheral vascular disease, saphenous vein quality) and extrinsic characteristics (elective or urgent). Additionally, the characteristics of the coronary lesion, such as the degree of stenosis, the minimum lumen diameter, and the fractional flow reserve, affect the choice of conduits.
Even after the graft decision has been made, the manner of using the graft is still controversial. Some researchers propose in situ graft use, while others prefer free grafts. When using a free graft, the choice must be made between reimplanting the graft in the aorta or in another graft in a composite fashion. The composite assembly is also debated: is it preferable to use a T or a Y shape, and where do we perform this crucial anastomosis? When used in situ, there is no evidence indicating whether it is better to use the right internal thoracic artery (RITA) on the left anterior descending artery crossing the midline or through the transverse sinus to a first marginal. Moreover, some researchers suggest using one graft to only one distal anastomosis, while others prefer sequential anastomosis.
After patient characteristics, the most important consideration is the coronary lesion itself. Most of the surgical literature on graft patency is based on visual inspection for coronary lesion evaluation. Cardiologists have long tried to find a more accurate method to evaluate the severity of the coronary lesion (quantitative coronary angiography, fractional flow reserve); unfortunately, these methods have not been applied to the evaluation of graft performance.
Historically, graft patency evaluation has been performed for the saphenous vein graft (SVG) using the Fitzgabon classification. Since arterial conduits display a totally different endothelium response to shear stress and competition flow, these historical definitions of patency have become obsolete, leading to new concepts such as “graft functioning.”
Moreover, in contradiction to the evidence-based medical literature, there are few evidence-based cardiac surgery reports. Indeed, most of the graft patency literature is retrospective, with few systematic angiographic controls. Most control studies were symptoms-driven, leading to a false evaluation of the conduit performance. Recent studies have promoted the use of less-invasive graft evaluations without evidence of a good correlation with gold-standard angiography.
In addition to all rational discussion of graft choice, there is one last powerful factor: “The Surgeon.” Each cardiac surgeon with a given mentorship background is more prone to use a certain graft type even if there is evidence of better performance using a different graft. The best example of this phenomenon is the 5% rate of bilateral internal thoracic artery (BITA) grafting in the United States, whereas some American surgeons have dedicated their careers to convincing their colleagues that two internal thoracic arteries (ITAs) are better than one. On the other hand, some European surgeons use BITA at all ages (even older than 80 years) without careful evaluation of the coronary lesion and knowing that the benefit of the second mammary only appears after 10 years.
STRATEGY OF GRAFT ASSEMBLY FOR THE LEFT CORONARY SYSTEMBilateral Internal Thoracic Arteries, the Best Grafts?BITAs have clearly demonstrated their superiority over all other types of grafts in terms of patency, freedom from arteriosclerosis, and survival benefit for revascularization of the left coronary system. Rankin et al.1 studied the 20-year clinical benefits of BITA vs single ITA grafting. Seven to 10 years of follow-up were required before the advantages of BITA grafting were apparent, but from 10 to 20 years, the benefits of BITA are statistically and clinically significant. However, even if the ideal graft has clearly been demonstrated, the method of use is still controversial. Therefore, several configurations of BITA have been proposed to achieve complete left-sided myocardial revascularization.
There are 3 major assembly strategies for revascularizing the left coronary system with BITA: a) in situ left internal thoracic artery (LITA) to the left anterior descending territory and in situ RITA to the circumflex territory through the transverse sinus; b) in situ RITA to the left anterior descending and in situ LITA to the circumflex territory, and c) in situ LITA to the left anterior descending territory and free RITA implanted in a Y or T fashion into the LITA.
In Situ Left Internal Thoracic Artery to the Left Anterior Descending Territory and in Situ Right Internal Thoracic Artery to the Circumflex Territory Through the Transverse SinusThe advantages of this configuration are the following: a) each ITA is used in situ and therefore is able to consistently provide sufficient blood flow to each target vessel, and b) the RITA does not cross the midline of the chest in front of the aorta in case of redo sternotomy or aortic valve surgery.2 The disadvantages are as follows: a) when using the RITA through the transverse sinus, the length used to cross the chest to reach the circumflex territory enables the grafting of medial or distal marginal branches; b) in order to reach the proximal marginal or intermediate artery, the entire length of the RITA until its distal bifurcation is necessary. Therefore, the RITA anastomotic site is often small and very muscular, which has been identified as a factor leading to worse patency3; c) the possibility of making sequential anastomosis is poor due to the short RITA length, and d) if multiple marginal branches must be grafted, it is necessary to use another graft, such as the radial artery (RA) graft or the SVG.
In Situ Right Internal Thoracic Artery to the Left Anterior Descending and in Situ Left Internal Thoracic Artery to the Circumflex TerritoryThe advantages of this configuration are the following: a) each ITA is used in situ and therefore is able to consistently provide sufficient blood flow to each target vessel, and b) the LITA can revascularize several branches of the circumflex system, avoiding the need for an accessory graft for the circumflex system. The disadvantages are as follows: a) the RITA crosses the midline of the chest in front of the aorta, increasing the risk of graft injury during redo or aortic valve surgery, and b) if the left anterior descending is very diseased and needs to be grafted distally, this is not always possible with the RITA4.
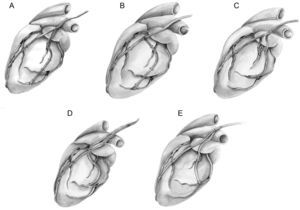
In Situ Left Internal Thoracic Artery to the Left Anterior Descending Territory and Free Right Internal Thoracic Artery Implanted in A Y or T Fashion Into The Left Internal Thoracic ArteryComposite Y-graft configurations using the free RITA graft anastomosed proximally to the LITA have been widely used.5 The advantages of this configuration are several: a) this assembly allows a complete myocardial revascularization with two ITAs without a complementary graft6; b) RITA does not cross the midline of the chest in front of the aorta in case of redo sternotomy or aortic valve surgery, and c) there is often no need to completely harvest the RITA in this assembly, decreasing the risk of wound complications by keeping a substantial residual blood supply in the lower half of the right hemisternum.7 The disadvantages are the following: a) the ability of this arrangement to completely revascularize the coronary system, including the right coronary artery (RCA), has been debated. It has been questioned whether a single ITA can consistently provide sufficient blood flow, especially in the composite Y-graft to 3 territories; b) there is a theoretical possibility of a “steal phenomenon” (the diversion of blood flow from a high resistance to a low resistance branch during hyperemia), resulting in a fall in the perfusion pressure in one branch of the Y assembly during periods of maximal myocardial blood flow demand8,9; c) the BITA Y configuration has the increased risk of competitive flow in the composite graft compared with the in situ graft. Indeed, in such assemblies the mechanism of competitive flow is more complex than in the individual graft, where the interaction is only between the proximal inflow and the distal anastomosis outflow. In this sequential composite bypass, the interaction is also between all the anastomosed branches within the composite graft, leading to a phasic delay between the pressure waves in the grafts and in the coronary arteries, especially in the more distant ones such as the RCA. Nakajima et al.10 and our group11 have reported that the most significant predictor of competitive flow and graft occlusion is the presence of a moderately stenotic branch in the RCA territory. We analyzed the functioning of the RITA in a Y-graft configuration, and found the RITA function was significantly improved when used on several branches of the circumflex artery or on a severely narrowed (>70%) first circumflex and was negatively affected by the presence of a grafted RCA. We believe that the RITA used in a Y-graft configuration has the same interaction with the RCA and the right gastro-epiploic artery (RGEA), and d) the RITA arrangement for the intermediate branch grafting is problematic when multiple grafting is necessary on the lateral wall of the heart. Indeed, we found that grafting a coronary branch in the intermediate region had a negative prognostic influence on RITA function. Previously, we systematically performed the proximal composite anastomoses of the free RITA on the LITA in a Y fashion on the posterior side (fascia), and the distal sequential circumflex anastomosis in a diamond-shaped fashion. The last anastomosis of the RITA was performed in a T or T/L fashion depending on the length between this anastomosis and the last diamond-shaped anastomosis.11 This arrangement may cause kinking of the intermediate anastomosis, especially if the proximal Y anastomosis is performed near the pulmonary artery region or inside of the pericardium (Fig. 1A). As a result of this finding, we changed our practice and prefer to perform a proximal T anastomosis on the LITA (Fig. 1B), use a second small Y-graft to prevent seagull kinking (Fig. 1C), perform the proximal composite anastomosis of a free RITA very high on the LITA in order to obtain a smooth curve of the RITA to the intermediate branch (Fig. 1D), and perform a L/L (latero-lateral) anastomosis on the intermediate and a T anastomosis on the circumflex artery (Fig. 1E). The latter 3 solutions decrease the available length of the RITA to the distal marginal branches or RCA branches.
A: Angulation of the right internal thoracic artery on the intermediate branch is not perpendicular. B: Proximal T anastomoses on the left internal thoracic artery. C: Use of a second small Y-graft. D: Proximal composite anastomose of a free right internal thoracic artery on the left internal thoracic artery very high on the left internal thoracic artery. E: Latero-lateral anastomose on the intermediate branch.
Initially described in 1973,12 the RA grafting was soon abandoned because reports documented dismal early angiographic outcomes.12 However, improvements in graft harvesting techniques, avoidance of mechanical dilation, new preservation methods, and the use of postoperative calcium channel blocker therapy to prevent early vasospasm led to a resurgence in the use of the artery as a bypass graft in the 1990s.
The biggest randomized trial13,14 comparing RA graft and SVG patency reached the following conclusions: a) analysis of the RA graft failures revealed that RA graft patency was more likely in patients with progressively severe proximal stenoses, suggesting that RA grafting should not be considered in the setting of moderate (<90% proximal obstruction) or questionably severe target vessel obstructions; b) In female patients, the rate of saphenous vein occlusion at 1 year was significantly higher compared to RA grafts. This finding was not true in males, and c) there is an impact of concomitant “peripheral vascular disease” on the fate of RA grafts and the lack of any comparable effect on SVG patency. This study reemphasized that RA graft patency was better than SVG patency at 1 year. This advantage, however, was reliably seen only when the degree of proximal coronary artery stenosis was severe (>90%). In addition, patients with peripheral vascular disease had poorer RA patency than was observed in SVG. The authors noted improved RA vs SVG patency in women, as opposed to poorer RA patency in women in other reports. These findings can be explained because the RA is a muscular artery, susceptible to vasospasm. Numerous studies have revealed that the RA has a higher receptor-mediated contractility than the ITA.15 These aspects of the RA may contribute to its vasospastic characteristics and its weakness in competing with native coronary flow in case of moderate coronary stenosis.
Strategies to Revascularize the Right Coronary SystemLeft and right coronary systems exhibit distinct physiological flow patterns and different patterns of atheromatous disease, which, for example, may account for poorer patency of an in situ RITA grafted to the RCA compared with a left-sided target. Therefore, selection of the optimal conduit for the RCA or its branches cannot simply be extrapolated from data arising from left-sided or mixed targets.
The conduits used for revascularization of the RCA system include the saphenous vein, the RITA in situ or in a Y-composite arrangement, the free RA, and the RGEA. The influence of clinical results on the choice of conduit type remains unclear, and the complementary conduit of choice to this system has yet to be determined. No superior patency rate has been established for any one of these grafts to the RCA.16,17 The use of the RA or RGEA as the conduit for moderate stenosis of the RCA is limited due to its association with a high risk of graft failure owing to competitive flow. Limited flow capacity of the RGEA has also been reported.18 One evaluation of the SVG to the RCA territory revealed surprisingly good clinical and angiographic results in long-term follow-up.19
Hadinata et al.20 reported absolute patency rates of 83.6% for the RA and 76.5% for the SVG targeted on the RCA; these patencies are lower than the latest reported Radial Artery Patency and Clinical Outcomes (RAPCO) rates (90% for RA and 87% for SVG) in a 5-year average follow-up.21 Possible explanations for these differences include the following: a) longer mean duration of follow-up may lead to a later drop off in patency; b) The RCA is likely a smaller target artery and has a smaller territory of runoff than the majority of the RAPCO study grafts, most of which were directed to the left side (indicating that the RCA was thought by the surgeon to be a lower-order target), and c) unlike in the main trial analyses of protocol-directed angiography results, Hadinata et al. included a mix of both protocol-directed and symptom-directed angiography. This last observation explains in part why patency rates presented in this series are lower than reported elsewhere from the RAPCO data set, as symptom-directed studies may underestimate overall patency rates.20
Pevni et al17 studied 1000 consecutive patients who underwent left-sided revascularization with BITA. In 231 patients, RCA grafting was performed with free RITA, in 246 with RGEA, in 142 with SVG, and 381 did not receive any graft to the RCA (no-graft group). At mid-term follow-up, similar 6-year survival rates and similar return of angina rates were observed in all 4 groups, suggesting no observable benefit of free RITA or RGEA grafts over SVGs.
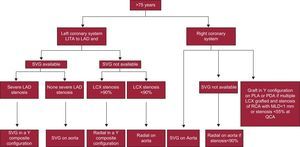
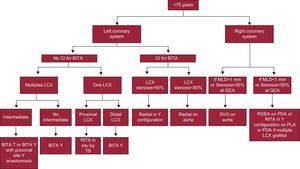
For these reasons we have modified our choice of the second graft and thereby our grafting strategy for the right coronary system (Figs. 2 and 3).
Decision tree for patients older than 75 years. LAD, left anterior descending; LCX, left circumflex artery; LITA, left internal thoracic artery; MLD, minimum lumen diameter, PDA, posterior descending artery; PLA, posterolateral artery, QCA, quantitative coronary angiography; RCA, right coronary artery; SVG, saphenous vein graft.
Decision tree for patients younger than 75 years. BITA, bilateral internal thoracic arteries; CI, contraindication; LCX, left circumflex artery; MLD, minimum lumen diameter; PDA, posterior descending artery; PLA, posterolateral artery; RGEA, right gastro-epiploic artery; RITA, right internal thoracic artery; SVG, saphenous vein graft; TS, transverse sinus; QCA, quantitative coronary angiography.
ITAs have proven their superiority over all other conduits for the left coronary system mainly because they are the best-equipped arterial conduit to withstand the competition flow, thanks to their endothelial function. RA and RGEA are more sensitive to flow competition because of their anatomy, vasomotion, and endothelial function. Therefore these 2 arterial conduits should be used only in case of very critical lesion to avoid graft occlusion. Only the SVG conduit is not significantly affected by flow competition, mainly due to its absence of resistivity and its common reimplantation in the aorta.
Graft configuration is the second important factor influencing the functionality equation between graft flow and native coronary flow and thereby the choice of graft. The more distal to the aorta, the higher the risk of competition flow. Therefore, composite grafting should be reserved for severely stenotic coronary targets, especially if multiple distal anastomoses must be performed on the latero-inferior wall of the heart. The use of more accurate tools such as the fractional flow reserve to evaluate the stenosis severity should be the milestone in the future of coronary surgery in order to decrease the rate of flow competition and improve arterial grafting functionality.
CONFLICTS OF INTERESTNone declared.