Keywords
INTRODUCTION
The increasing use of iodinated contrast medium (CM) in radiological procedures, particularly during coronary angiography, has raised concerns about the growing incidence of a potential complication known as contrast-induced nephropathy (CIN).
As the third leading cause of hospital-acquired acute renal failure,1 CIN has a serious impact on patient outcomes including death, myocardial infarction and stroke, especially in those requiring dialysis after the procedure.2-5 Overall incidence of CIN following coronary angiography varies greatly (2%-50%), with baseline presence of chronic renal disease (CRD) and diabetes mellitus being the most important risk factors.2 Chronic renal disease has been considered both necessary and sufficient to cause CIN, whereas diabetes has been seen as amplifying it.6 Other risk factors such as hypotension, intra-aortic balloon pump, congestive heart failure, age >75 years, anemia, and CM volume should also be considered.6,7
Moreover, CIN represents one of the few hospital-acquired types of kidney failure physicians can prevent. The cornerstone of prophylaxis in both high-and low-risk patients is adequate parenteral volume repletion. Other pharmacological measures—such as prophylactic n-acetylcysteine (NAC) administration in patients with CRD because NAC is both an antioxidant and vasodilator—have been evaluated in randomized, controlled trials with greatly varied results.8-23
Hypothetically, the direct effect of NAC on renal tubular creatinine secretion or muscle metabolism could influence the protective effect of NAC against CIN incidence when evaluating serum creatinine concentration (SrCr).24 Although other renal injury markers have been proposed,25,26 a recent paper concluded that SrCr is a good clinical marker of CIN detection.27
Consequently, we conducted a randomized prospective study to test the hypothesis that intravenous NAC administration would prevent CIN in high-risk coronary patients with CRD who undergo coronary angiography. Other short- and long-term clinical effects were also evaluated.
METHODS
Patients
The Hospital Clínico Universitario de Valencia, Spain, is a 600-bed tertiary referral center. The study prospectively enrolled 90 patients admitted to the Coronary Unit (CU) with acute coronary sydrome and DRD and referred for cardiac catheterization.
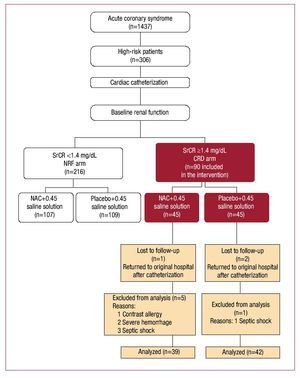
To fulfill CONSORT recommendations,28 Figure 1 presents a flowchart of patient enrollments. We included coronary patients considered high-risk following diagnosis of angina at rest or post-myocardial infarction, or receiving thrombolytic therapy after failed revascularization and indicated for emergency cardiac catheterization. We defined CRD as stable SrCr ≥1.4 mg/dL (123.76 µmol/L)9 or <60 mL/min creatinine clearance calculated with the Cockcroft-Gault formula.29 Major exclusion criteria were hemodynamic instability (systolic blood pressure <90 mm Hg), known NAC or contrast agent allergies, untreated gastrointestinal bleeding, and/ or previous antibiotic treatment with theophylline, mannitol or nephrotoxic drugs.
Figure 1. Main protocol study design. Flowchart of chronic renal disease (CDR) arm of the main study; n, number of patients in brackets; NAC, N-acetylcysteine; NRF arm, normal renal function arm of the main study; SrCr, serum creatinine concentration. (Reprinted from Carbonell N, et al,30 with permission from Elsevier).
Data was collected from March 1, 2002 to December 31, 2006 and—except for 1-year mortality—collection ended upon discharge. The study protocol was approved by the local ethics committee and informed consent obtained from all patients or their relatives.
Study Design
This study presents the CRD arm of the main protocol study design (Figure 1). Results on the normal renal function (NRF) population, (the NRF arm), appear elsewhere.30 The CRD arm is a randomized, double-blind, placebo-controlled trial. Patients were randomly assigned to intravenous NAC (600 mg diluted in 50 mL of 0.9% saline) or to a placebo (50 mL of 0.9% saline solution) for 30 minutes twice daily for a total of 4 doses. Hypotonic intravenous saline solution was administered at a rate of 1 mL/Kg/h at the first 6 h before the procedure and was maintained for 12 h after contrast-dosing in both CRD study groups. Randomization was with computer-generated random numbers. Analysis was based on intention-to-treat.
Levels of SrCr, plasma concentration of urea and electrolytes were recorded during the 24 h prior to catheterization and considered baseline references. Measurements were repeated at 24 and 48 h after contrast administration. The primary endpoint was development of CIN, defined as an acute increase in SrCr of ≥0.5 mg/dL (44 µmol/L) or >25% increase above baseline at 48 h after contrast administration. Secondary outcomes were CIN-induced need for dialysis, CU mortality, and the combination of inhospital and 1-year mortality.
Side effects during NAC administration, such as vomiting, hypotension, bronchospasm, fever, dizziness and flushing, or itching, were recorded. In the catheterization laboratory, non-ionic, lowosmolality CM iopromide (Ultravist, 370 mg iodine/mL, Schering AG, Germany) was used in all patients.
Statistical Analysis
Statistical analysis used SPSS 9.0. Continuous data are expressed as mean (SD) or median (range) and categorical data as percentages. Continuous variables with normal distributions were analyzed with the Student t test and categorical variables with Fisher's exact test and c2. The Kolmogorov-Smirnov test showed SrCr was not normally distributed so the non-parametric Wilcoxon and Mann-Whitney U tests were used to assess differences between the groups. We calculated the SrCr increase/decrease as SrCr at 48 h minus baseline SrCr divided by baseline SrCr.
The alpha significance level was set at .05 and 1-b power at .80. We calculated that 45 patients per CRD-arm group were required for an acetylcysteine versus placebo study. Sample size was estimated from the C4-Study Design Pack program (Copyright GlaxoWellcome, SA) and was a projection based on Tepel et al8 and Goldenberg et al,21 which demonstrated the probability of developing CIN in the NAC group was P1=.02, and in the placebo group P2=.21.
Risk factors were evaluated with univariate and multivariate analysis (multiple logistic stepwise regression). The relationship between potential confounding factors and composite endpoint development was determined by constructing a Cox Proportional Hazards model.
RESULTS
Full data were obtained and analyzed for 81 patients (NAC=39; placebo=42); 9 of the original randomized 90 were excluded due to partial lack of data (Figure 1). Three patients were lost in the follow-up because they returned to their original hospitals after the procedure; 6 were excluded (1 developed an unknown allergy to CM; 2 presented hemorrhage secondary to thrombolytic therapy; 3 developed septic shock postprocedure). Baseline clinical and biochemical characteristics of the patient population are in Table 1. The 2 groups were comparable in terms of demographic features, cardiovascular risk factors, concomitant medication, analytical data, and data related to catheterization. Fifteen of the 29 patients (51.7%) diagnosed with ST-segment elevation myocardial infarction received thrombolytic therapy; 3 (20%) required rescue angioplasty. Table 1 shows the CM volume delivered in each study group. This was significantly higher in patients needing percutaneous interventions versus patients undergoing diagnostic coronary angiography (204.41 [23.7] vs 124.6 [11.8] [P=.001]). However, the 2 main study groups were comparable in terms of the procedure employed (Table 1).
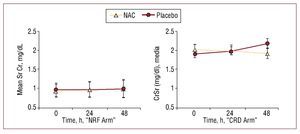
Overall CIN incidence was 14.8% (12): 5.1% (2) in the NAC group and 23.8% (10) in the placebo group (P=.027, OR=0.17 [0.03-0.84]) (Table 2). No NAC side effects were found. Mean SrCr decreased from 2.01 (0.77) to 1.90 (0.83) mg/dL at 48 h after CM administration in NAC-treated patients, whereas it increased from 1.87 (0.70) to 2.15 (0.94) mg/dL in the placebo group (P=NS) (Figure 2). No significant changes were recorded in serum urea nitrogen concentration at 48 h after cardiac catheterization which fell from 80 (36) to 76 (37) mg/dL in the NIC group but rose from 67 (23) to 71 (27) mg/dL in the placebo group.
Median CU stay was 5 (1-20) and 4 (2-27) days in the NAC and placebo groups respectively, and median hospital stay was 10 (1-42) and 10 (2-73) days, respectively (P=.20). One CIN patient required dialysis while in CU (1.2%). This patient belonged to the placebo group and died 1 year later. The other CIN patient death occurred at 1 month, also in the placebo group (overall CIN-group mortality was 17%). Three CIN and 2 placebo group patients (7.7% vs 4.8%) died in the CU (P=.18). Stepwise logistic regression identified NAC as a protective factor for the composite short-term endpoint of CIN, need-for-dialysis and CU mortality (OR=0.20, 95% confidence interval, 0.04-0.97; P=.04). Coronary artery bypass graft surgery was performed during the hospitalization in 7 NAC and 12 placebo group patients (17.9% vs 28.6%) (P=.25). However, we found no differences in mid- and long-term mortality (Table 2). Cardiac death was diagnosed in 1 of the 4 patients who died after discharge.
DISCUSSION
The increased use of iodinated CM in coronary angiography still causes concern over the development of CIN, especially in urgent cases when prophylactic hydration is not always possible, or in patients with a high-risk CIN profile (ie, patients presenting baseline CRD or congestive heart failure). Because of its favorable side-effect profile, low cost and some positive results in randomized trials, NAC has earned its place in clinical practice with these high-risk patient groups. However, evidence of its renoprotective effect is limited and the issue remains unresolved.31 Therefore, we conducted a randomized, prospective study to test the hypothesis that intravenous NAC administration would prevent CIN in high-risk coronary patients with CRD undergoing coronary angiography.
One of the outstanding findings of the current study has been its confirmation of NAC's benefits in CIN prevention, based on reducing SrCr levels. We found significantly low CIN incidence when NAC was administered prior to coronary angiography in patients with CRD (5.1% vs 23.8%), which coincides with results reported elsewhere.16 However, these results contrast with the lack of additional benefits from saline hydration previously found in coronary patients with normal baseline renal function (CIN incidence 10.3% vs 10.1% in NAC and placebo groups, respectively).30 This underscores the fact that NAC's preventive effect only relates to high-risk patients with baseline CRD.
Furthermore, in the CRD arm of the main study, mean SrCr decreased at 24 and 48 h after CM when NAC was employed. This is contrary to the trend observed in the NRF arm (Figure 2). The difference in creatinine level behavior found in the NAC group of both arms of the main protocol strongly argues in favor using creatinine as a marker to assess NAC's direct renoprotective effect. We believe our study casts serious doubts on the conclusions drawn by Hoffmann et al.24 Their study enrolled healthy volunteers who received NAC (without contrast dye) and reported the absence of NAC's glomerular effect on SrCr was justified by the lack of any effect on the cystatin C level at 4 h after administration. In the same year, Rickli et al25 compared the kinetic differences of cystatin C and SrCr at 5, 24, and 48 h after radiocontrast was used in patients with normal to subnormal glomerular filtration rates. Their major findings were that the cystatin C increase peaked at 24 h after angiography, with a delayed increase in SrCr. They found no significant change by comparison with baseline in SrCr or cystatin C at 5 h after CM administration. Hoffman et al did not present biochemical marker data at 24 hours postprocedure, which appears to be the crucial moment to detect changes in cystatin C. Furthermore, Levin A et al26 have recently found a biological effect of NAC defined by albumin excretion, suggesting this may attenuate contrast-induced glomerular or tubular injury, independently of any effect on creatinine.
Figure 2. Changes in mean serum creatinine (SrCr) concentrations in both arms of the study. CRD arm indicates chronic renal disease arm of the main study; NAC, N-acetylcysteine; NRF arm, normal renal function arm of the main study. Values are expressed as mean (SD). The Wilcoxon-Mann-Whitney U test was used to assess differences between NAC and placebo groups in both arms of the main study. (NRF arm results reprinted from Carbonell N, et al,30 with permission from Elsevier.)
We concur with Marenzi et al27 who consider NAC should have a protective effect when oxidative stress induced by ischemia and reperfusion is present, rather than when it is absent, as in healthy patients.24 The antioxidant effect of NAC may be the physiological explanation for the renal protection it affords.
On the other hand, the clinical benefit obtained with the new protocol for the intravenous administration of NAC in high-risk patients with CRD, which aroused controversy when it was used in patients with normal renal function,30 is important for several reasons: a) the apparent lack of side-effects by comparison with the 14% of adverse reactions found by Baker et al when administering large intravenous doses13; b) the possible use of NAC in patients who present in an emergency (earlier pharmacokinetic studies establish a metabolite half-life of 6 h)32; c) its low oral bioavailability (20%); and d) the potential for patient rejection of oral NAC because of its unpleasant taste.
The second major finding of this study has been the clinical benefit associated with NAC administration in short-term follow-up. Until a few years ago there had been just one randomized study showing a significant reduction in hospitalization after CM in the group receiving oral NAC.11 Large case series and cohort studies2,4 have shown patients with CIN rarely need dialysis after coronary intervention (<1% vs 14.6% when dialysis is not required) but found an association with high in-hospital mortality, while most prospective and randomized studies have failed to demonstrate any clinical benefit of NAC when analyzing these outcomes. Mehran et al7 developed a simple risk score for CIN prediction and observed an exponential 13% increase in overall CIN incidence and increases in dialysis and 1-year mortality with the increased risk score. They demonstrated 0.12% and 1.09% risk of dialysis associated with 14% and 26% risk of developing CIN, respectively. These data could justify the absence of events in most studies and coincide with our results: overall CIN incidence was 14.8% and 1 patient with CIN in the placebo group required dialysis in the CU (1.2%); CIN incidence was 23.8% in this group.
Furthermore, we have identified NAC as a short-term protective factor when analyzing the composite endpoint of CIN, hospital mortality and need for urgent dialysis. Marenzi et al27 enrolled consecutive patients with myocardial infarction undergoing primary angioplasty and recently demonstrated improved in-hospital evolution, as well as a dose-dependent reduction in the severity of CIN. However, mainly in the control group, they found a higher percentage of CIN incidence and short-term events than earlier studies did, with mortality much higher than the 4%-5% rate typically reported in large registries.33 Although our study did not enroll exactly the same population, our 4.8% CU mortality in the placebo group is consistent with registries that reflect real clinical practice. However, the clinical benefit of NAC has not translated into a fall in adverse outcomes in long-term follow-up.
Limitations
This study was designed to determine CIN incidence. The sample size has insufficient statistical value to assess differences in morbidity and mortality. Although the relatively small sample size calls for caution in interpreting results, we would point out that it was determined by applying research design software using previously published results. Another limitation is the fact we did not measure cystatin C as a surrogate marker of glomerular filtration. It seems to reflect CM-induced changes in kidney function better than SrCr, which could lead to erroneous conclusions concerning NAC benefit. However, results recently obtained by Kimmel et al34 are preliminary, and still have limited value due to the small sample size.
CONCLUSIONS
In the absence of a multicenter study, we recommend the use of intravenous NAC prior to coronary angiography in high-risk patients with SrCr ≥1.4 mg/dL, especially when hydration is impossible because of their clinical characteristics.
ABBREVIATIONS
CIN: contrast-induced nephropathy
CM: contrast medium
CRF: chronic renal failure
NAC: N-acetylcysteine
NRF: normal renal function
SrCr: serum creatinine concentration
SEE ARTICLE ON PAGES 9-11
Correspondence: Dra. N. Carbonell.
Unidad Coronaria y de Medicina Intensiva. Hospital Clínico Universitario. Av. Blasco Ibáñez, 17. 46010 Valencia. España.
E-mail: edurnecarbonell@yahoo.es
Received November 10, 2008.
Accepted for publication August 5, 2009.