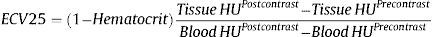Myocardial interstitial fibrosis, a hallmark of hypertrophic cardiomyopathy (HCM), has been proposed as an arrhythmic substrate. Fibrosis is associated with increased extracellular volume (ECV), which can be quantified by computed tomography (CT). We aimed to analyze the association between CT-determined ECV and malignant ventricular arrhythmias.
MethodsA retrospective case-control observational study was conducted in HCM patients with implantable cardioverter-defibrillator, undergoing a CT-protocol with continuous iodine contrast infusion to determine equilibrium ECV. Left ventricular septal and lateral CT-determined ECV was compared between prespecified cases (malignant arrhythmia any time before CT scan) and controls (no prior malignant arrhythmias) and among ECV tertiles.
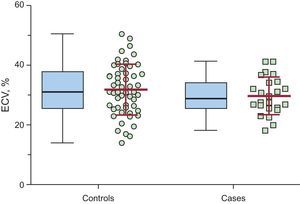
ResultsA total of 78 implantable cardioverter-defibrillator HCM patients were included; 24 were women, with a mean age of 52.1 ± 15.6 years. Mean ECV ± standard deviation in the septal left ventricular wall and was 29.8% ± 6.3% in cases (n = 24) vs 31.9% ± 8.5% in controls (n = 54); P = .282. Mean ECV in the lateral wall was 24.5% ± 6.8% in cases vs 28.2% ± 7.4% in controls; P = .043. On comparison of the entire population according to septal ECV tertiles, no significant differences were found in the number of patients receiving appropriate shocks. Conversely, we found a trend (P = .056) for a higher number of patients receiving appropriate shocks in the lateral ECV lowest tertile.
ConclusionsExtracellular volume was not increased in implantable cardioverter-defibrillator HCM patients with malignant ventricular arrhythmias vs those without arrhythmias. Our findings do not support the use of ECV (a surrogate of diffuse fibrosis) as a predictor of arrhythmias in high-risk HCM patients.
Keywords
Hypertrophic cardiomyopathy (HCM) is a genetically transmitted form of cardiomyopathy with an estimated prevalence of 1/500 inhabitants in the general population.1–6 The disease can have a favorable outcome,7 especially with contemporary management strategies5; however, sudden cardiac death (SCD) remains a risk, and estimation of SCD risk is a rapidly evolving field of research.
The main strategy used to prevent SCD in high-risk HCM patients is the insertion of an implantable cardioverter-defibrillator (ICD).8–10 However, in most HCM patients the implanted ICD is never used. Furthermore, ICD insertion carries a risk of inappropriate shocks and other complications. There is therefore a need for better tools to stratify arrhythmia risk in HCM.
Ventricular arrhythmias leading to SCD in HCM are thought to develop from myocardial fibrosis.11 Some studies have linked ventricular arrhythmias to focal fibrosis, assessed from the presence and extent of late gadolinium enhancement on cardiac magnetic resonance (CMR).12–14 However, this relationship is not considered powerful enough to support ICD implantation as a primary prevention measure in American or European guidelines.15,16 Late gadolinium enhancement-CMR does not detect diffuse fibrosis; however, postmortem histology shows that diffuse fibrosis is more prevalent after SCD in HCM patients than in deaths not linked to a cardiovascular cause or in aths in patients with left ventricular hypertrophy of hypertensive origin, suggesting that it is a proarrhythmic substrate.11,17–20 To date, few studies have been designed to evaluate the association between malignant ventricular arrhythmias and diffuse fibrosis as detected noninvasively.21,22
Myocardial fibrosis is associated with increased extracellular volume (ECV), which can be quantified by CMR or computed tomography (CT).23,24 The aim of our study was to determine whether quantification of ECV by CT, as a surrogate measure of diffuse fibrosis, could distinguish between the presence or absence of malignant ventricular arrhythmias in HCM patients fitted with an ICD (ICD-HCM patients).
METHODSA retrospective case-control observational study was performed in ICD-HCM patients. Between November 2013 and February 2015, 78 ICD-HCM patients (> 18 years old without contraindications for contrast CT) were recruited at 5 Spanish cardiomyopathy units (Puerta de Hierro-Majadahonda, Madrid, n = 24; La Fe, Valencia, n = 15; Son Llatzer, Palma de Mallorca, n = 17; Clínico San Carlos, Madrid, n = 10; 12 de Octubre, Madrid, n = 12). The study was approved by the local ethics committees. All patients had been previously implanted with an ICD according to current risk stratification guidelines.16,25,26 All patients gave written informed consent.
Case/Control Prespecified GroupsCases consisted of HCM patients with an ICD implanted for secondary prevention or those with an ICD implanted for primary prevention and receiving documented appropriate ICD therapy (antitachycardia pacing or shock). Control patients were defined as HCM patients with an ICD implanted for primary prevention but with no history of ICD therapy at the time of enrolment.
Computed Tomography ProtocolExtracellular volume was quantified through CT data acquired with 2 CT scanners: an ICT 256 (Philips, Best, The Netherlands) at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid (n = 61 participants) and a Lightspeed VCT 64 Slice CT scanner (General Electric, United States) at Son Llatzer Hospital, Mallorca (n = 17 participants). Before CT scanning, patients underwent tests to verify heart rate, blood pressure, and cardiac rhythm (sinus rhythm, atrial fibrillation, or pacemaker), and blood was drawn for a hematocrit test. The CT imaging protocol consisted of scout sequences and 2 acquisitions (precontrast and postcontrast) with coverage in the z direction of 160mm (to provide maximum coverage of the left ventricle). Computed tomography data were acquired prospectively at 70% of the RR interval. Postcontrast acquisitions were performed 25minutes after initiation of infusion with iodinated contrast agent (Omnipaque 300mg L/mL, GE Healthcare). The contrast was infused rapidly with a CT injector (Medrad Stellant for scans at the CNIC; Ulrich Medical missouri for scans at Son Llatzer Hospital) at a rate of 3mL/s to a total volume of 1mL/kg. Upon completion of the rapid infusion, continuous perfusion was initiated with an infusion pump at 1.88mL/h/kg and continued for 25minutes (Hospira PlumA+ at the CNIC; Braun Space Infusomat at Son Llatzer Hospital).24 For safety reasons, the maximum volume of administered contrast agent was set at 200mL. The absorbed radiation dose was expressed as the dose-length product (mGy•cm), and the effective radiation dose was calculated as dose-length product * 0.014, and expressed in milliSieverts.27
Image AnalysisImage analysis was conducted at the CNIC core imaging laboratory by observers blinded to clinical data. Extended Work Station (Philips, Best, The Netherlands) was used to reconstruct 5-mm slices. The slice with the best quality, as defined by the absence of beam-hardening artifacts from the ICD lead, and enough myocardium and blood was selected for further analyses. A region of interest (ROI) was traced in the interventricular septum at the area of maximum myocardial thickness. When focal fibrosis was evident,28 it was included in the ROI with the rest of the septal tissue. Similarly, a ROI was traced inside the left ventricular blood pool (Figure 1). Finally, a lateral ROI was positioned on the lateral wall. Regions of interest were positioned identically for the precontrast and postcontrast acquisitions.
Two computed tomography images with optimized window settings to highlight contrast between myocardial and blood attenuation are exhibited. In all images, the region of interest is positioned in the septum (white circle), and in blood pool (black circle). Precontrast acquisition image and acquisition 25minutes after initiation of pump infusion are shown.
Extracellular volume was estimated after 25minutes of pump infusion using the formula24:
Implantable Cardioverter-defibrillators and Clinical Follow-upEvents recorded at ICD follow-up were documented (specifically the number and dates of appropriate shock therapies, inappropriate shock therapies, and antitachycardia pacing; the presence of paroxysmal or permanent atrial fibrillation was also documented). Data from the most recent ICD follow-up were used to determine the presence or absence of appropriate ICD therapies. The following echocardiography parameters were recorded: maximum interventricular septum thickness, parasternal left atrial diameter, and maximum gradient in the left ventricular outflow tract. The New York Heart Association functional class was recorded at the time of the CT scan. Five-year SCD risk for HCM (%) was estimated when possible, according to the method of O’Mahony et al., which evaluates echocardiogram data, family history, and clinical data at the date of ICD implantation (except for current age).29 Risk was not calculated for secondary prevention.
Statistical AnalysisStatistical comparisons were made with IBM SPSS Statistics V.22. Qualitative variables are expressed as No. (%). Quantitative variables are expressed as mean ± standard deviation for data with a normal distribution or as median (interquartile range [IQR]) when the sample had a nonnormal distribution. Qualitative variables were compared by the chi-square test and quantitative variables were compared by the Student t test. The nonparametric Mann-Whitney U test was used when needed. For tertile comparisons ANOVA (analysis of variance) and the chi-square test (Mantel Haenszel test for linear trend) where applied when appropriate. The intraclass correlation coefficient was calculated to test intraobserver and interobserver variation. Statistical differences were considered significant at P < .05.
RESULTSA total of 78 patients were included (24 [30.8%] women, mean age 52 ± 16 years), 24 cases and 54 controls. Demographic characteristics are presented in Table 1 and Table 1 of the supplementary material. Most patients were in a good functional class at the time of inclusion: 71% were in New York Heart Association class I, with just 3% in New York Heart Association classes III-IV. Of the total population, 5% were on cardiac resynchronization therapy and 12% were on diuretics. Median maximal wall thickness was 20.7mm (IQR, 17.1-25.0mm) measured by CT, compared with 22.0mm (IQR, 18.0-28.3mm) on the last echo exam before inclusion. A total of 67% of patients were under treatment with beta-blockers and 13% were under treatment with amiodarone. Excluding the secondary prevention patients, the median 5-year SCD risk according to European Society of Cardiology (ESC) guidelines29 was 3.9% (IQR, 2.9%-6.3%) without statistical differences regarding the appearance of malignant ventricular arrhythmias after ICD implantation.
Patient Characteristics
| Total population (N = 78) | Cases (n = 24) | Controls patients (n = 54) | P | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, y | 52.1 ± 15.6 | 51.2 ± 19.4 | 52.5 ± 13.8 | .775 |
| Female, | 24 (31) | 5 (21) | 19 (35) | .205 |
| BSA, m2 | 1.90 ± 0.19 | 1.95 ± 0.22 | 1.88 ± 0.18 | .152 |
| Time from ICD implantation to CT, y | 4.8 [2.2-6.2] | 5.1 [3.4-6.1] | 3.4 [2.0-6.8] | .335 |
| Laboratory | ||||
| Hematocrit, % | 46.6 ± 5.0 | 48.0 ± 5.6 | 45.9 ± 4.5 | .069 |
| Creatinine, mg/dL | 0.93 ± 0.23 | 0.96 ± 0.17 | 0.92 ± 0.25 | .556 |
| Maximum wall thickness on index CT exam, mm | 20.7 [17.1-25.0] | 21.0 [18.6-25.0] | 20.3 [17.0-25.0] | .236 |
| Maximum wall thickness on last preenrolment echo exam, mm | 22.0 [18.0-28.3] | 22.5 [18.3-28.0] | 21.5 [18.0-29.0) | .825 |
| Left atrial diameter on last echo exam, mm | 42.0 [38.3-48.0] | 43.0 [40.0-48.0] | 41.0 [37.5-48.5] | .317 |
| 5-year SCD risk, %a | 3.9 [2.9-6.3] | 4.5 [3.1-6.6]b | 3.8 [2.6-5.9] | .598 |
| NYHA functional class | ||||
| I | 55 (71) | 17 (71) | 38 (70) | .715 |
| II | 21 (27) | 6 (25) | 15 (28) | |
| III-IV | 2 (3) | 1 (4) | 1 (2) | |
BSA, body surface area; CT, computed tomography; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association; SCD, sudden cardiac death.
Unless otherwise indicated, values are expressed as mean ± standard deviation, median [interquartile range] or No. (%).
In 17 (21.8%) of our population, the risk score was not calculated due to a lack of any of the necessary data. Additional clinical characteristics are shown in Table 1 of the supplementary material.
Within the cases population (N = 24), 14 patients had an ICD implanted for secondary prevention. The remaining 10 patients had an ICD implanted for primary prevention and documented malignant ventricular arrhythmias treated with appropriate ICD therapy during follow-up. Cases with ICD implemented on secondary prevention had any ICD therapy (antitachycardia pacing or shock) earlier than those on primary prevention. Data on ICD therapies in the whole study population are summarized in Table 2 of the supplementary material.
The mean volume of iodine contrast administered to patients was similar in cases and controls (146.7mL vs 138.4mL; P = .147). The median effective radiation dose was the same for both study groups (4.5 mSv vs 4.5 mSv for case patients and controls, P = .295). Attenuation values for each of the compartments are summarized in Table 2, with the noise level indicated by the standard deviation. Additional CT procedure-related data are shown in Table 3 of the supplementary material. The differences in septal ECV in patients with or without atrial fibrillation and type of atrial fibrillation are shown in Table 4 of the supplementary material and Table 5 of the supplementary material, respectively.
Computed Tomography Results
| Total population (N = 78) | Case patients (n = 24) | Control patients (n = 54) | P (cases vs controls) | |
|---|---|---|---|---|
| ECV and attenuation, septal wall | ||||
| ECV mean, % | 31.3 ± 7.9 | 29.8 ± 6.3 | 31.9 ± 8.5 | .282 |
| Myocardium precontrast, HU | 46.8 ± 5.9 | 47.7 ± 5.8 | 46.4 ± 5.9 | .370 |
| Blood precontrast, HU | 43.9 ± 7.0 | 44.1 ± 6.7 | 43.9 ± 7.2 | .910 |
| Myocardium 25min, HU | 73.0 ± 7.0 | 74.5 ± 6.6 | 72.3 ± 7.1 | .184 |
| Blood 25min, HU | 87.8 ± 7.8 | 89.5 ± 9.0 | 87.1 ± 7.2 | .218 |
| ECV, lateral wall | ||||
| ECV lateral wall mean, % | 27.1 ± 7.4 | 24.5 ± 6.8 | 28.2 ± 7.4 | .043 |
ECV, extracellular volume.
Data are expressed as mean ± standard deviation.
Additional computed tomography data are presented in Table 2 of the supplementary material.
Mean ECV in the interventricular septum (primary outcome measure) was 31.3% ± 7.9% in the entire cohort, 29.8% ± 6.3% in cases, and 31.9% ± 8.5% in controls, P = .282 (Table 2, Figure 2). The ECV in the lateral wall was 27.1% ± 7.4%, 24.5% ± 6.8% in cases vs 28.2% ± 7.4% in controls, P = .043. Extracellular volume in the interventricular septum was significantly higher than that calculated in the lateral left ventricular wall: 31.3% ± 7.9% vs 27.1% ± 7.4%, P < .001. The ratio “septal ECV/lateral wall ECV” (a surrogate of left ventricular ECV asymmetry) was nonsignificantly higher in cases patients (1.32 ± 0.11) than in controls (1.17 ± 0.31), P = .133.
For the 64 participants with ICD in primary prevention, ECV was also compared between cases and controls. Extracellular volume was 31.9 ± 8.5% for controls (n = 54) and was 32.6 ± 6.8 (P = .81) for cases (n = 10).
For all parameters (myocardial and blood attenuation both precontrast and postcontrast), intraclass correlation coefficient values were between 0.8 and 0.9 with significant P values in all cases, demonstrating the good reproducibility of the CT data (Table 3).
Reproducibility of Extracellular Volume on Computed Tomography
| Interclass correlation | P | |
|---|---|---|
| Intraobserver variability (n = 78) | ||
| Myocardial attenuation precontrast, HU | 0.846 | < .001 |
| Blood attenuation precontrast, HU | 0.875 | < .001 |
| Myocardial attenuation postcontrast 25min, HU | 0.918 | < .001 |
| Blood attenuation post contrast 25min, HU | 0.902 | < .001 |
| Interobserver variability (n = 61) | ||
| Myocardial attenuation precontrast, HU | 0.914 | < .001 |
| Blood attenuation precontrast, HU | 0.873 | < .001 |
| Myocardial attenuation postcontrast 25min, HU | 0.814 | < .001 |
| Blood attenuation postcontrast 25min, HU | 0.806 | < .001 |
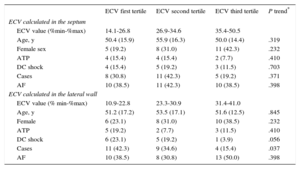
The entire population was divided into septal and lateral ECV tertiles and ICD therapies were compared among groups. There was no linear trend in in the number of patients receiving appropriate shocks among septal ECV tertiles: 4 (15.1%), 5 (19.2%) and 3 (11.5%) in the lowest, intermediate and highest septal ECV tertiles respectively (P = .703). Conversely, we found a nonsignificant trend among ECV lateral tertiles with a higher incidence of appropriate ICD shocks in patients in the lowest lateral ECV tertile compared with those in the other tertiles (P = .056). A statistically significant linear trend among ECV lateral tertiles was found on comparison of cases vs controls, as previously defined (P = .037) (Table 4).
Tertiles of Extracellular Volume Calculated on Septum and Lateral Wall
| ECV first tertile | ECV second tertile | ECV third tertile | P trend* | |
|---|---|---|---|---|
| ECV calculated in the septum | ||||
| ECV value (%min-%max) | 14.1-26.8 | 26.9-34.6 | 35.4-50.5 | |
| Age, y | 50.4 (15.9) | 55.9 (16.3) | 50.0 (14.4) | .319 |
| Female sex | 5 (19.2) | 8 (31.0) | 11 (42.3) | .232 |
| ATP | 4 (15.4) | 4 (15.4) | 2 (7.7) | .410 |
| DC shock | 4 (15.4) | 5 (19.2) | 3 (11.5) | .703 |
| Cases | 8 (30.8) | 11 (42.3) | 5 (19.2) | .371 |
| AF | 10 (38.5) | 11 (42.3) | 10 (38.5) | .398 |
| ECV calculated in the lateral wall | ||||
| ECV value (% min-%max) | 10.9-22.8 | 23.3-30.9 | 31.4-41.0 | |
| Age, y | 51.2 (17.2) | 53.5 (17.1) | 51.6 (12.5) | .845 |
| Female | 6 (23.1) | 8 (31.0) | 10 (38.5) | .232 |
| ATP | 5 (19.2) | 2 (7.7) | 3 (11.5) | .410 |
| DC shock | 6 (23.1) | 5 (19.2) | 1 (3.9) | .056 |
| Cases | 11 (42.3) | 9 (34.6) | 4 (15.4) | .037 |
| AF | 10 (38.5) | 8 (30.8) | 13 (50.0) | .398 |
AF, atrial fibrillation, ATP, antitachycardia pacing; DC, direct current; ECV, extracellular volume.
Unless otherwise indicated, values are expressed as No. (%).
This is the first study to evaluate the association between diffuse myocardial fibrosis and malignant ventricular arrhythmias in high-risk HCM patients with ICD. We tested the hypothesis that CT-detected ECV, a surrogate for diffuse myocardial fibrosis, would be greater in high-risk HCM patients with malignant ventricular arrhythmic events than in those without. To do this, we examined HCM patients with a previously implanted ICD, dividing the population according to the presence or absence of arrhythmias (either preinsertion, ie, secondary prevention, or postinsertion, ie, appropriate ICD therapy in an individual with an ICD implanted as a primary prevention strategy). Extracellular volume in ICD-HCM patients with malignant ventricular arrhythmias was not increased compared with those without malignant ventricular arrhythmias. From another perspective, when the full cohort was divided by tertiles, we found a trend to a higher incidence of appropriate shocks in patients in the lowest ECV tertile. Our results thus do not support the hypothesis and suggest that quantification of ECV would not improve arrhythmia risk prediction in HCM patients.
Myocardial fibrosis, with increased myocardial collagen matrix deposition, is a recognizd phenomenon during the natural history of HCM, detected during autopsy of young victims of HCM-related sudden death.11 In HCM patients, increased collagen synthesis can be tracked from increased circulating levels of type I procollagen C-terminal propeptide.30 The advent of late gadolinium enhancement-CMR allowed the study of macroscopic fibrosis and led to suggestions of an association with lethal arrhythmic events in HCM patients. A study of 1293 HCM patients found the extent of late gadolinium enhancement to be a good predictor of SCD, showing a 40% increase in relative SCD risk for every 10% increase in late gadolinium enhancement over a median follow-up of 3.3 years.12 However, a meta-analysis including 1063 patients from 4 studies found the association between late gadolinium enhancement and sudden death to be nonsignificant over a mean follow-up of 3.1 years31; moreover, an analysis of 711 HCM patients similarly found no statistically significant association between the presence and quantity of fibrosis (late gadolinium enhancement + areas on CMR) and SCD over a median follow-up of 3.5 years.13 Thus, while there is a plausible link between fibrosis and arrhythmia risk, there is no definite association between focal macroscopic fibrosis detected by late gadolinium enhancement-CMR and malignant ventricular arrhythmias or SCD. Reflecting this situation, risk stratification in current guidelines for ICD implantation does not include the presence of late gadolinium enhancement only as a minor criterion, considering it potentially useful in patient selection,16 as it was included in the previous guidelines as a minor criteria.15 The recent advent of imaging techniques able to quantify diffuse microscopic fibrosis23 has allowed reassessment of the hypothesis that fibrosis is a risk marker in HCM patients.
In our population, 64 patients had an ICD implanted for primary prevention, 10 of whom (15.6%) had ≥ 1 appropriate therapy over a median follow-up of 4.8 years (3.3%/y). This is similar to the published figure of 3.6% per year,32 and clearly higher than the calculated median risk of 3.9% per 5 years (0.8%/y) in the ESC guidelines algorithm.29 However, it is important to mention that not all appropriate therapies would have been lifesaving treatments, because it is well known that some ventricular arrhythmias are self-terminating without any ICD intervention, and thus the percentage of appropriate ICD therapies is not directly equivalent to the risk of sudden death. However, there is a striking difference observed between the estimated risk of sudden death with the ESC algorithm and the much higher percentage of appropriate ICD therapies. In this regard, missing information should be considered. In 21.8% of our population, risk score was not calculated due to a lack of any of the necessary data. The percentage of appropriate therapies in our primary prevention population included in the cases group (3.3%/y) was slightly higher than the established risk of sudden death (6% 5-year risk, 1.2%/y) for ICD implantation according to current ESC guidelines.16 Of the 14 patients in our study who had an ICD implanted for secondary prevention, 8 (57.1%) had ≥ 1 documented appropriate therapy event over a mean follow-up of 5.0 years (11.4%/y), similar to the 10.6%/y recorded in other cohorts.32
Computed Tomography Validity for Extracellular Volume CalculationThe landmark study by Flett et al.23 introduced noninvasive equilibrium-contrast CMR as a method for studying diffuse fibrosis, previously only accessible by histology. The original study population included 8 HCM patients; however, subsequent reports by the same group examined larger numbers of HCM patients.33 Once the usefulness of ECV for measuring diffuse myocardial fibrosis was established, attention turned to alternative imaging techniques. A comparison of the performance of CT and CMR for measuring ECV showed excellent correlation between the 2 methods in populations with aortic stenosis or cardiac amyloidosis.24,34 Normal ECV values in healthy individuals reported in CMR studies are around 25%.33 There are no data on the CT-based ECV values in healthy individuals.
Compared with CMR, CT has been less extensively investigated as a method to quantify ECV, and ours is the first published report using CT to measure ECV in HCM patients. Computed tomography has a lower resolution than CMR and exposes patients to radiation; conversely, unlike CMR, its use is safe in patients with ICDs or other implanted cardiac devices. Previous experience with CT used to quantify ECV includes evaluation of diffuse fibrosis in heart failure patients35 and in patients with aortic stenosis24 or amyloidosis.34 In the 2 latter studies, validation of CT against CMR showed comparable results with the 2 techniques. The precision of for ECV and its safety in patients with an ICD prompted us to use this method to evaluate diffuse fibrosis in a high-risk population of patients who already had an implanted ICD. This enabled us to study the association between diffuse fibrosis and arrhythmia risk in the best-case scenario.
Role of Extracellular Volume to Detect Risk of Malignant Ventricular Arrhythmias in Patients With Implantable Cardioverter-defibrillatorThe absence of any association between ECV and arrhythmias in our study has several possible explanations. The first possibility is that increased fibrosis in HCM might not increase the risk of developing malignant arrhythmias. In this interpretation, our results would fit with those of previous studies showing no significant association between late gadolinium enhancement and arrhythmia risk.13,31 In our study, we did not include a group of non-HCM healthy participants, and thus cannot confirm that ECV was increased in our HCM population; however, the mean ECV (31%) for the full cohort is similar to reported values for HCM patients with CMR and is higher than those for control groups,18,21,33,36,37 suggesting that there was significant diffuse fibrosis in our population. The second possibility is that ECV is not an accurate marker of myocardial fibrosis. Previous studies showed a reasonable correlation between ECV and collagen content in myocardial biopsies23; however, collagen is just one extracellular component, and the extracellular compartment is also affected by edema (acute myocardial infarction), inflammatory infiltration, and other myocardial conditions.38,39 The increased ECV in ICD-HCM patients might therefore be driven not only by diffuse fibrosis but also by these other components. A third possibility relates to the classification of participants according to their history of malignant ventricular arrhythmia; some patients with high ECV but no history of arrhythmia may go on to develop arrhythmias in the future. This possible explanation for the neutral results will be addressed in future follow-up studies in our population. The main reason we designed the study this way was the relatively low incidence of SCD in unselected HCM populations.5 By selecting a high-risk HCM population we aimed to maximize the chance of identifying differences in ECV between patients developing arrhythmias and those without malignant ventricular events. We cannot, however, exclude the possibility that fibrosis increases arrhythmia risk only in the unselected HCM population and not in a very high-risk population.
More difficult to interpret is the significant linear trend found for more cases in the lowest lateral wall ECV tertile. This result is in contradiction to our prespecified hypothesis of more ECV being associated with patients developing arrhythmias. Interestingly, we found that case patients had a higher (albeit nonsignificant) “septal ECV/lateral wall ECV” ratio than controls. One possible interpretation is that, since all HCM patients have a high ECV in the septal wall, those with high lateral wall ECV values (thus less left ventricular ECV asymmetry) could be associated with a reduced incidence of ventricular arrhythmias. These data should be interpreted with caution since they are merely hypothesis-generating and purely speculative at this moment.
LimitationsThis was a retrospective case-controls study and thus we cannot rule out the possibility that some patients classified as controls will develop an arrhythmic event in the near future. In this regard, we plan to follow-up the participants enrolled in our study to document whether any controls develop an event and to evaluate the extent of ECV in these individuals. Although the sample size was limited, the results clearly show that the working hypothesis was not correct and therefore the potential lack of power played no role in the results observed. Due to the unfeasibility of CMR studies in this population, total myocardial mass calculations and late gadolinium enhancement data were not available (limited acquisition in CT for safety reasons also prevents left ventricular mass calculation). For the same reason, we do not know how many patients had macroscopic fibrosis. We therefore cannot exclude the possibility that some ROIs included areas of macroscopic fibrosis. However, if this were the case, we anticipate that macroscopic fibrosis would be more pronounced in patients affected by arrhythmias. Therefore, any bias would have skewed the results toward higher ECV values in cases and not controls, and would thus not have contributed to the negative results presented here.
CONCLUSIONSAs measured by CT in equilibrium, ECV in HCM patients with documented malignant ventricular arrhythmias is not increased compared with HCM patients without malignant ventricular arrhythmias. Longer follow-up studies are warranted to investigate the potential of ECV to improve risk prediction of malignant ventricular arrhythmias in HCM.
FUNDINGThis work was funded by the RIC (Red de Investigación Cardiovascular) of the Spanish Ministry of Health, familial cardiomyopathy program (RD 12/0042/0054 to B. Ibáñez; RD 12/0042/066 to P. García-Pavía; RD 12/0042/0069 to T. Ripoll-Vera; RD12/0042/0036, RD06/0003/0009 to REDINSCOR [Red Española de Insuficiencia Cardiaca]). This work was supported by the Plan Estatal de I+D+I 2013-2016 – ERDF (European Regional Development Fund) “A way of making Europe”. This study forms part of a MRA (Master Research Agreement) between CNIC and Philips Healthcare. The CNIC is supported by the Spanish Ministry of Economy and Competitiveness and the Pro-CNIC Foundation and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505).
CONFLICTS OF INTERESTJ. Sánchez-González is Philips employee.
- –
Sudden cardiac death remains a risk for the HCM population. Tools to quantify this risk are incomplete.
- –
Myocardial fibrosis is associated with increased myocardial ECV, which can be quantified by magnetic resonance or CT.
- –
Link between ECV and SCD in HCM has not been well established.
- –
Diffuse fibrosis in HCM measured through CT ECV is not increased in high-risk HCM patients with arrhythmic events compared to high-risk HCM patients without arrhythmic events.
- –
The involvement of diffuse fibrosis in the development of malignant ventricular arrhythmias in HCM patients needs further research.
We thank Noemi Escalera for coordinating the study, Angel Macías and Braulio Pérez-Asenjo for image acquisition, and Maite D. Rodríguez for taking care of patient wellbeing during the study. R. Fernández-Jiménez is a recipient of a FICNIC fellowship from the Fundació Jesús Serra, the FIC (Fundación Interhospitalaria de Investigación Cardiovascular), and the CNIC.