To the Editor:
Inhibitors of mTOR are drugs that have recently been introduced in the field of cardiac transplant. Therefore, their side effects have not been completely documented. The most frequent are leucopoenia, dyslipemia, and infections. Their incidence appears to be related with the dosage.1-3 The bilateral edemas are a known complication,4 but its unilateral manifestation has been described only rarely.5 We present the case of a patient who developed this complication when treated with both everolimus and sirolimus.
Male patient, 68 years of age, recipient of heart transplant in 1996 due to dilated myocardiopathy of enolic origin. OKT-3 induction therapy was administered for 7 days, and the maintenance consisted of cyclosporine (100 mg/12 h), azathioprine (100 mg/24 h), and deflazacort (6 mg/24 h). Subsequent evolution was satisfactory, without acute rejection, infections, or relevant complications.
In May 2004, he underwent an operation for an epithelioma in the parietal region, for which he needed a skin graft.
Subsequently, new cutaneous tumours appeared, leading to the decision to substitute the azathioprine with everolimus (0.75 mg/12 h) and to reduce the cyclosporine dosage (75 mg/12 h). The steroid dosage was not modified.
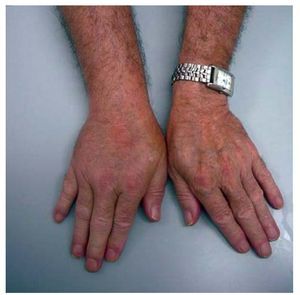
Three months after the change in treatment, the patient showed concentrations of cyclosporine and everolimus within the therapeutic range (124 ng/mL and 8 ng/mL). Examination showed definite cutaneous tumour remission, as well as significant nonpiting edema, which had been slowly progressing in the 2 previous months, in the right hand and forearm (Figure). Given the lack of venous system measurements, symptoms of infection and/or inflammation, or a history of lymphoedema, the decision was made to reduce the dose of everolimus (0.5 mg/12h) and run new clinical tests.
Figure. Edema in the right hand and forearm.
One month after the adjustment, with concentrations of everolimus at 5 ng/mL, the edema had been partially reduced, but was still significant and limiting for the patient. Consequently, it was decided to substitute the everolimus with sirolimus (1 mg/12 h). A few days later, the patient experienced a new increase in the edema with sirolimus levels of 10 ng/mL, which led to a return to the original treatment regime. Two months after that change, there was full recovery, without subsequent relapse of the manifestation. New neoplastic cutaneous lesions appeared, which required exeresis and clinical follow-up.
Due to their antiproliferative effects and low nephrotoxicity, mTOR inhibitors have a wide range of possibilities in the field of cardiac transplants. Peripheral edemas are not infrequent, occurring in about 55% of the cases,6 and they are usually controlled with low doses of furosemide accompanied by reducing the immunosuppressant.4 Pathophysiology seems to be due to altered lymphatic drainage,7,8 although a certain degree of proteinuria9 may also be involved.
This case presents several interesting aspects. Firstly, the unilateral location, then the relation between the symptom and the dosage, and lastly, the fact that it was associated with everolimus and worsened with sirolimus, which implies a physiological effect due to the class, not the molecule.