Data are lacking on the long-term prognosis of stable ischemic heart disease (SIHD). Our aim was to analyze long-term survival in patients with SIHD and to identify predictors of mortality.
MethodsA total of 1268 outpatients with SIHD were recruited in this single-center prospective cohort study from January 2000 to February 2004. Cardiovascular and all-cause death during follow-up were registered. All-cause and cardiovascular mortality rates were compared with those in the Spanish population adjusted by age, sex, and year. Predictors of these events were investigated.
ResultsThe mean age was 68±10 years and 73% of the patients were male. After a follow-up lasting up to 17 years (median 11 years), 629 (50%) patients died. Independent predictors of all-cause mortality were age (HR, 1.08; 95%CI, 1.07-1.11; P <.001), diabetes (HR, 1.36; 95%CI, 1.14-1.63; P <.001), resting heart rate (HR, 1.01; 95%CI, 1.00-1.02; P <.001), atrial fibrillation (HR, 1.61; 95%CI, 1.22-2.14; P=.001), electrocardiographic changes (HR, 1.23; 95%CI, 1.02-1.49; P=.02) and active smoking (HR, 1.85; 95%CI, 1.31-2.80; P=.001). All-cause mortality and cardiovascular mortality rates were significantly higher in the sample than in the general Spanish population (47.81/1000 patients/y vs 36.29/1000 patients/y (standardized mortality rate, 1.31; 95%CI, 1.21-1.41) and 15.25/1000 patients/y vs 6.94/1000 patients/y (standardized mortality rate, 2.19; 95%CI, 1.88-2.50, respectively).
ConclusionsThe mortality rate was higher in this sample of patients with SIHD than in the general population. Several clinical variables can identify patients at higher risk of death during follow-up.
Keywords
Cardiovascular disease is a major cause of death in Spain. According to data from the National Institute of Statistics, in 2015 there were 124 197 deaths,1 accounting for 29.4% of all deaths in Spain. Ischemic heart disease represents a large part of the cardiovascular disease spectrum: in Spain, 33 769 persons died in 2015 due to ischemic heart disease.1
Improvements in the treatment of acute coronary syndrome in recent decades has prolonged the survival of affected patients and has increased the prevalence of stable ischemic heart disease (SIHD).2 Among coronary conditions, the prognosis of SIHD has historically received less attention in research in our setting. Consequently, the available information on the long-term prognosis of these patients has been extrapolated from studies conducted in other countries and in other time periods.3–7
Our group has already published several articles on the prognostic impact of resting heart rate (HR)8 and blood pressure in patients with SIHD9 and on the prognosis of older patients with this condition.10
The aim of the present study was to investigate very long-term survival of a contemporary Spanish cohort of SIHD patients taken from daily clinical practice to compare all-cause and cardiovascular mortality rates with those observed in the Spanish general population and to identify predictors of all-cause and cardiovascular mortality.
METHODSThe CICCOR (Chronic Ischemic Heart Disease of Cordoba) registry is an observational, prospective, single-center cohort study to investigate the prognosis of SIHD.8–10
From January 2000 to February 2004, the study prospectively included all SIHD patients who came to 2 general cardiology outpatient offices at the hospital, referred by primary care physicians or emergency departments or for checkup after hospitalization in cardiology or internal medicine.
Patients were diagnosed with SIHD if they met 1 or more of the following inclusion criteria: history of acute coronary syndrome (unstable angina or acute myocardial infarction) or surgical or percutaneous coronary revascularization at least 3 months before inclusion; history of chest pain during stress test, myocardial perfusion imaging, or stress echocardiogram consistent with ischemia; or coronary angiography with stenosis >70% of the luminal diameter of an epicardial vessel, with no severe valve disease. Patients were excluded only if they declined to participate in the study.
Information on demographics, medical history, physical examination, and additional tests were collected at baseline. Abnormal electrocardiography was defined as the presence of left bundle-branch block, right bundle-branch block, pathologic Q wave, ST-segment depression> 1mm, or negative T wave in 2 or more contiguous leads. Cardiomegaly was considered to be cardiothoracic ratio> 0.5 in a previously performed posteroanterior chest radiograph.
Patients received treatment and follow-up at the discretion of their attending cardiologists in accordance with the clinical practice guidelines of the scientific societies in effect at the time.11–13 The study met the Helsinki guidelines for medical studies, and all patients gave written informed consent for inclusion and follow-up.
The main aim of the study was to investigate all-cause and cardiovascular mortality. To do this, the study identified the vital status of each patient between 1 June 2016 and 31 December 2016.
The data search included medical histories, primary care contact, and tele phone interviews when necessary to minimize losses to follow-up. The causes of death were collected from the medical history of patients who died in the hospital and from information provided by relatives or primary care physicians responsible for those who died outside a hospital. Cardiovascular death was considered to be death caused by acute coronary syndrome, acute aortic syndrome, heart failure, or stroke. There was no systematic retrieval of death records.
The all-cause and cardiovascular mortality rates of the sample were calculated for each age group, sex, and year up to 2015, with all living patients included in the denominator and all deaths for each age group and sex in each year included in the numerator. Mortality rates were calculated on a yearly basis as well as for the entire period studied. Last, the all-cause and cardiovascular mortality rates of the national, provincial, and sample populations studied were compared by age groups for calendar years and for the entire study period. To do this, the National Institute of Statistics1 was asked for national microdata on the cause of death for the study period. These data were then analyzed by an outside statistical service to calculate mortality rates and standardized mortality ratios.
In the statistical analysis, the normal distribution of quantitative data was confirmed by the Kolmogorov-Smirnov test; parametric quantitative data are expressed as mean±standard deviation and nonparametric data as median [interquartile range, p25-p75]. Qualitative variables are expressed as percentages. The Student t test or Mann-Whitney U test was used to compare quantitative variables, as appropriate, and the chi-square test was used to compare qualitative variables, using the Fisher exact test when necessary. Univariate associations of baseline parameters with all-cause and cardiovascular mortality parameters were studied by the Cox proportional hazards analysis. Kaplan-Meier curves were used to plot mortality during follow-up among subgroups that were independent predictors. P values <.05 were considered significant. Last, multivariate models were used with the Cox proportional hazards model. The proportional hazards assumption was verified by a plot method (logarithm-minus-logarithm plots). The models were initially fitted using all variables showing differences with P <.15. Variables with no statistical significance were removed by backward elimination, which finally included independent predictors. The results are expressed as hazard ratios (HR) with the respective 95% confidence intervals (95%CI). Left ventricular ejection fraction (LVEF) and cardiomegaly on chest radiography were not included in the final model because more than 10% of values had been lost. Likewise, medical treatment variables were not included in the final analysis due to the difficulty of eliminating associated biases. However, additional analyses were performed to include these variables and others with > 10% lost values, due to their prognostic importance in the scientific literature.
All statistical analyses were performed using IBM SPSS Statistics, version 21.0 (IBM Corp.; Armonk, New York).
RESULTSBaseline CharacteristicsA total of 1268 patients were included, and the median age was 68 [60-74] years. Almost 3 of every 4 (73%) patients included were men. The median time from the baseline coronary event until inclusion was 24 [6-63] months.
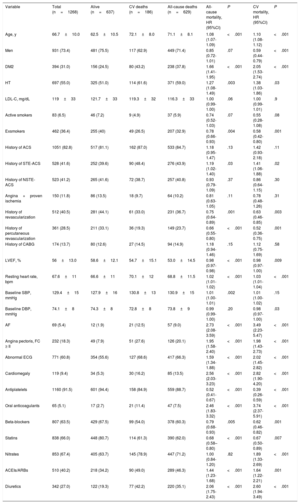
A total of 512 (40.5%) patients underwent revascularization before inclusion. Most (81.7%) patients were asymptomatic at the time of recruitment. The percentage of patients with a previous acute coronary event was high (1051 [82.8%]). Regarding the prevalence of cardiovascular risk factors at study initiation, around one third of patients had type 2 diabetes mellitus (DM2), and more than half had hypertension. Mean low-density lipoprotein cholesterol was 119±33 mg/dL. A total of 44% of the sample had been smokers. At inclusion, 1225 (96.6%) patients were receiving antithrombotic therapy and 65 (5.1%) were receiving oral anticoagulants. Statins were used by 66% of patients. The baseline characteristics of the sample are listed in Table 1.
Baseline Characteristics of the Series and Predictors of All-cause and Cardiovascular Mortality in the Univariate Analysis
| Variable | Total (n=1268) | Alive (n=637) | CV deaths (n=186) | All-cause deaths (n=629) | All-cause mortality, HR (95%CI) | P | CV mortality, HR (95%CI) | P |
|---|---|---|---|---|---|---|---|---|
| Age, y | 66.7±10.0 | 62.5±10.5 | 72.1±8.0 | 71.1±8.1 | 1.08 (1.07-1.09) | <.001 | 1.10 (1.08-1.12) | <.001 |
| Men | 931 (73.4) | 481 (75.5) | 117 (62.9) | 449 (71.4) | 0.85 (0.72-1.01) | .07 | 0.59 (0.44-0.79) | <.001 |
| DM2 | 394 (31.0) | 156 (24.5) | 80 (43.2) | 238 (37.8) | 1.66 (1.41-1.95) | <.001 | 2.05 (1.53-2.74) | <.001 |
| HT | 697 (55.0) | 325 (51.0) | 114 (61.6) | 371 (59.0) | 1.27 (1.08-1.49) | .003 | 1.38 (1.03-1.86) | .03 |
| LDL-C, mg/dL | 119±33 | 121.7±33 | 119.3±32 | 116.3±33 | 1.00 (0.99-1.00) | .06 | 1.00 (0.99-1.01) | .9 |
| Active smokers | 83 (6.5) | 46 (7.2) | 9 (4.9) | 37 (5.9) | 0.74 (0.52-1.03) | .07 | 0.55 (0.28-1.08) | .08 |
| Exsmokers | 462 (36.4) | 255 (40) | 49 (26.5) | 207 (32.9) | 0.78 (0.66-0.93) | .004 | 0.58 (0.42-0.80) | .001 |
| History of ACS | 1051 (82.8) | 517 (81.1) | 162 (87.0) | 533 (84.7) | 1.18 (0.95-1.47) | .13 | 1.42 (0.93-2.18) | .11 |
| History of STE-ACS | 528 (41.6) | 252 (39.6) | 90 (48.4) | 276 (43.9) | 1.19 (1.02-1.40) | .03 | 1.41 (1.06-1.88) | .02 |
| History of NSTE-ACS | 523 (41.2) | 265 (41.6) | 72 (38.7) | 257 (40.8) | 0.93 (0.79-1.09) | .37 | 0.86 (0.64-1.15) | .30 |
| Angina+proven ischemia | 150 (11.8) | 86 (13.5) | 18 (9.7) | 64 (10.2) | 0.81 (0.63-1.05) | .11 | 0.78 (0.48-1.26) | .31 |
| History of revascularization | 512 (40.5) | 281 (44.1) | 61 (33.0) | 231 (36.7) | 0.75 (0.64-0.89) | .001 | 0.63 (0.46-0.85) | .003 |
| History of percutaneous revascularization | 361 (28.5) | 211 (33.1) | 36 (19.3) | 149 (23.7) | 0.66 (0.55-0.80) | <.001 | 0.52 (0.36-0.75) | .001 |
| History of CABG | 174 (13.7) | 80 (12.6) | 27 (14.5) | 94 (14.9) | 1.18 (0.94-1.46) | .15 | 1.12 (0.75-1.69) | .58 |
| LVEF, % | 56±13.0 | 58.6±12.1 | 54.7±15.1 | 53.0±14.5 | 0.98 (0.97-0.98) | <.001 | 0.98 (0.97-1.00) | .009 |
| Resting heart rate, bpm | 67.6±11 | 66.6±11 | 70.1±12 | 68.8±11.5 | 1.02 (1.01-1.02) | <.001 | 1.03 (1.01-1.04) | <.001 |
| Baseline SBP, mmHg | 129.4±15 | 127.9±16 | 130.8±13 | 130.9±15 | 1.01 (1.00-1.01) | .002 | 1.01 (1.00-1.02) | .15 |
| Baseline DBP, mmHg | 74.1±8 | 74.3±8 | 72.8±8 | 73.8±9 | 0.99 (0.99-1.00) | .20 | 0.98 (0.97-1.00) | .03 |
| AF | 69 (5.4) | 12 (1.9) | 21 (12.5) | 57 (9.0) | 2.73 (2.08-3.59) | <.001 | 3.49 (2.23-5.47) | <.001 |
| Angina pectoris, FC ≥ II | 232 (18.3) | 49 (7.9) | 51 (27.6) | 126 (20.1) | 1.95 (1.58-2.40) | <.001 | 1.98 (1.43-2.73) | <.001 |
| Abnormal ECG | 771 (60.8) | 354 (55.6) | 127 (68.6) | 417 (66.3) | 1.59 (1.34-1.88) | <.001 | 2.02 (1.45-2.82) | <.001 |
| Cardiomegaly | 119 (9.4) | 34 (5.3) | 30 (16.2) | 85 (13.5) | 2.56 (2.03-3.23) | <.001 | 2.82 (1.90-4.20) | <.001 |
| Antiplatelets | 1160 (91.5) | 601 (94.4) | 158 (84.9) | 559 (88.7) | 0.52 (0.41-0.67) | <.001 | 0.39 (0.26-0.59) | <.001 |
| Oral anticoagulants | 65 (5.1) | 17 (2.7) | 21 (11.4) | 47 (7.5) | 2.46 (1.83-3.32) | <.001 | 3.74 (2.37-5.91) | <.001 |
| Beta-blockers | 807 (63.5) | 429 (67.5) | 99 (54.0) | 378 (60.3) | 0.79 (0.68-0.93) | .005 | 0.62 (0.46-0.82) | .001 |
| Statins | 838 (66.0) | 448 (80.7) | 114 (61.3) | 390 (62.0) | 0.68 (0.58–0.80) | <.001 | 0.67 (0.50-0.89) | .007 |
| Nitrates | 853 (67.4) | 405 (63.7) | 145 (78.9) | 447 (71.2) | 1.00 (0.84-1.20) | .82 | 1.89 (1.33-2.69) | <.001 |
| ACEIs/ARBs | 510 (40.2) | 218 (34.2) | 90 (49.0) | 289 (46.3) | 1.44 (1.23-1.68) | <.001 | 1.64 (1.22-2.21) | .001 |
| Diuretics | 342 (27.0) | 122 (19.3) | 77 (42.2) | 220 (35.1) | 2.06 (1.75-2.43) | <.001 | 2.60 (1.94-3.49) | <.001 |
95%CI, 95% confidence interval; ACEIs, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; AF, atrial fibrillation; ARBs, angiotensin II receptor antagonists; CABG, coronary artery bypass graft; CV, cardiovascular; DBP, diastolic blood pressure; DM2, type 2 diabetes mellitus; ECG, electrocardiogram; FC, functional class; HR, hazard ratio; HT, hypertension; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; NSTE-ACS, non–ST-elevation acute coronary syndrome; SBP, systolic blood pressure; STE-ACS, ST-segment elevation acute coronary syndrome.
The median follow-up was 11.2 [4-15] years (maximum follow-up, 17 years), with an observation period of 12 612 patient-years and only 2 patients lost to follow-up. A total of 629 (49% of all) patients died during follow-up; 252 patients (40% of nonsurvivors) died due to a noncardiovascular cause, whereas 186 deaths were due to a cardiovascular cause (30% of deaths). In 191 cases (30% of nonsurvivors), the cause of death could not be determined. The survival probabilities were 92%, 80%, 67%, 56%, and 45% at 3, 6, 9, 12, and 15 years of follow-up, respectively.
Predictors of Total MortalityAccording to the results of the univariate analysis, nonsurvivors were significantly older, were more likely to have hypertension, DM2, and atrial fibrillation (AF), and had higher systolic blood pressure and resting HR. Higher LVEF and a history of revascularization were associated with lower mortality during follow-up according to the univariate analysis. In addition, nonsurvivors initially were more likely to have angina pectoris in functional class ≥ II, an abnormal baseline electrocardiogram, and cardiomegaly on chest radiography. The use of anticoagulants, diuretics, and angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists was associated with higher all-cause mortality in the univariate analysis. However, antiplatelet, beta-blocker, and statin therapy was associated with lower mortality during follow-up. The results of the univariate analysis are listed in Table 1.
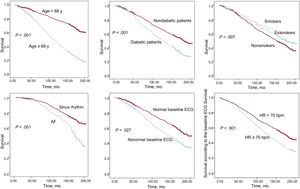
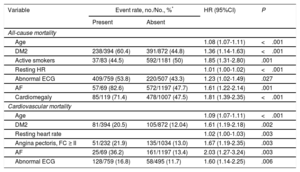
The variables independently associated with all-cause mortality by the multivariate analysis were age, DM2, active smoking, resting HR, abnormal baseline electrocardiogram, or diagnosed AF (Table 2, Figure 1). An additional analysis included medical treatment and revealed an independent association of diuretic therapy with mortality (HR=1.78; 95%CI, 1.42-2.22; P < .001). Likewise, although more than 10% of LVEF values had been lost (consequently, LVEF was not included in the final analysis), further analysis showed that it was independently associated with mortality (HR=0.99; 95%CI, 0.98-0.99; P < .008).
Independent Predictors of All-cause and Cardiovascular Mortality in the Final Multivariate Models
| Variable | Event rate, no./No., %* | HR (95%CI) | P | |
|---|---|---|---|---|
| Present | Absent | |||
| All-cause mortality | ||||
| Age | 1.08 (1.07-1.11) | <.001 | ||
| DM2 | 238/394 (60.4) | 391/872 (44.8) | 1.36 (1.14-1.63) | <.001 |
| Active smokers | 37/83 (44.5) | 592/1181 (50) | 1.85 (1.31-2.80) | .001 |
| Resting HR | 1.01 (1.00-1.02) | <.001 | ||
| Abnormal ECG | 409/759 (53.8) | 220/507 (43.3) | 1.23 (1.02-1.49) | .027 |
| AF | 57/69 (82.6) | 572/1197 (47.7) | 1.61 (1.22-2.14) | .001 |
| Cardiomegaly | 85/119 (71.4) | 478/1007 (47.5) | 1.81 (1.39-2.35) | <.001 |
| Cardiovascular mortality | ||||
| Age | 1.09 (1.07-1.11) | <.001 | ||
| DM2 | 81/394 (20.5) | 105/872 (12.04) | 1.61 (1.19-2.18) | .002 |
| Resting heart rate | 1.02 (1.00-1.03) | .003 | ||
| Angina pectoris, FC ≥ II | 51/232 (21.9) | 135/1034 (13.0) | 1.67 (1.19-2.35) | .003 |
| AF | 25/69 (36.2) | 161/1197 (13.4) | 2.03 (1.27-3.24) | .003 |
| Abnormal ECG | 128/759 (16.8) | 58/495 (11.7) | 1.60 (1.14-2.25) | .006 |
95%CI, 95% confidence interval; AF, atrial fibrillation; DM2, type 2 diabetes mellitus; ECG, electrocardiogram; FC, functional class; HR, hazard ratio.
In the univariate analysis, cardiovascular mortality was associated with angina pectoris in functional class ≥ II; a history of acute coronary syndrome, DM2, hypertension, or AF, and higher age or resting HR. In addition, an abnormal baseline electrocardiogram or cardiomegaly on chest radiography were also associated with higher cardiovascular mortality. Higher diastolic blood pressure at the first visit, male sex, higher LVEF, and a history of revascularization before inclusion were associated with lower cardiovascular mortality during follow-up. Treatment with diuretics, oral anticoagulants, or angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists was also associated with higher cardiovascular mortality in the univariate analysis. Conversely, patients who received antiplatelet, statin, and beta-blocker therapy had lower cardiovascular mortality than those not receiving such therapy.
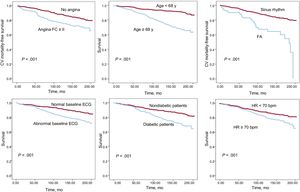
In the final multivariate model, higher cardiovascular mortality was independently associated with older age, higher resting HR, a history of DM2, angina pectoris in functional class ≥ II, or AF, and abnormal electrocardiogram (Table 2, Figure 2).
Further analyses showed that diuretic treatment was independently associated with cardiovascular mortality (HR=2.58; 95%CI, 1.81-3.69; P < .001), whereas LVEF did not affect mortality due to this cause during follow-up (HR=1.00; 95%CI, 0.99-1.01; P < .55).
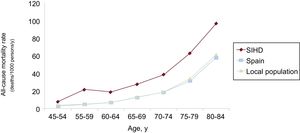
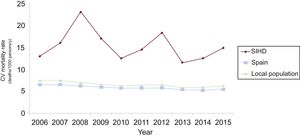
Comparison With the Spanish General PopulationThe gross yearly mortality rate of our population during the entire follow-up period was 47.81/1000 patients/y. A population designed with the same age and sex distribution to which year-adjusted mortality rates for the general population from the National Institute of Statistics were applied had an all-cause mortality rate of 36.29/1000 persons/y. The standardized mortality ratio was 1.31 (95%CI, 1.21-1.41).
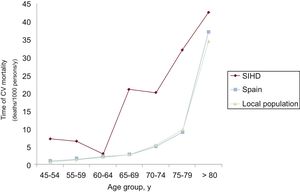
The yearly cardiovascular mortality rate of our population was around 15.25, compared with 6.94/1000 inhabitants/y in a population of similar age and sex to which the cardiovascular mortality rates for the general Spanish population were applied for this period. The standardized mortality ratio was 2.19 (95%CI, 1.88-2.50).
The results by age groups, sex, and year are shown in Figure 3, Figure 4, and Figure 5.
The most important finding of the present study is the high all-cause and cardiovascular mortality of a contemporary Spanish cohort of SIHD patients, significantly higher than that of the Spanish general population of the same age and sex.
Earlier studies to analyze the SIHD mortality, whether observational or post hoc analyses of clinical trials,4,14–18 generally reported all-cause mortality rates around 15.5-35.5/1000 patients/y, lower than those of the sample, and cardiovascular mortality rates around 4.6-20.0/1000 patients/y, similar to those observed in this study. Only the BEAUTIFUL study,19 which included patients with depressed LVEF, found a higher mortality rate.
Three possible reasons, among others, could explain the higher all-cause mortality observed in our series. First, the populations are different in terms of baseline characteristics, medical treatment, and revascularization rate. The CORONOR study reported a similar mortality rate to that of the general population, lower than the one observed in our study (33/1000 patients/y), despite a similar distribution in terms of age and cardiovascular risk factors; however, the percentage of patients with a history of acute coronary syndrome was lower (62% vs 83%), with 99% of patients already revascularized,16 compared with 40.5% in our sample. In addition, the all-cause mortality rate found in our study is also higher than that reported by the Spanish BARIHD study,17 at 32.5 deaths/1000 patients/y. In that study population, lipid and blood pressure control was better, and revascularization and statin use rates were higher (87% vs 66%). The recruitment time points could have been related to the various baseline characteristics and, consequently, the all-cause mortality of both samples. Last, SIHD patients in the REACH registry18 had a lower all-cause mortality rate (28.5/1000 patients/y). That series showed similar demographic characteristics to those of our study, although there was a lower rate of ischemic events (59%) and higher rates of percutaneous (42%) and surgical (32%) revascularization. In addition, the patients included were more likely to be receiving statin therapy (79%) and angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists (71%).
Second, the mean follow-up did not exceed 5 years in any of these studies. The long follow-up of our series may have allowed a more precise estimate of long-term mortality.
Last, geographic variations in all-cause mortality rates cannot be ruled out: the REACH study observed differences in mortality and cardiovascular events between various areas of the world,20 and regional differences in mortality due to coronary disease have also been described in Spain.21
Regarding cardiovascular mortality, it is noteworthy that only 30% of deaths in the sample were due to a cardiovascular cause, whereas studies such as BARIHD17 or REACH18 reported 64% and 56%, respectively. In this regard, 30% of deaths in this population were of unknown cause, and a large portion could be cardiovascular, which would yield similar proportions of cardiovascular deaths to those of the studies cited. In fact, previous studies have attributed deaths of unknown cause to cardiovascular mortality.18
Nevertheless, our population had a similar age and sex distribution to that obtained in other studies that have analyzed the prevalence of SIHD in Spain: the TRECE22 and REPAR23 registries, which investigated the baseline characteristics of patients with SIHD in Spain, had a mean age of 67 years and 71% and 80% of male sex, respectively, similar to the age of 66.7 years and 73% of male sex seen in our study population. However, the rate of previous revascularization in this population was lower than that of such studies (40% vs 70% and 57% in the TRECE and REPAR registries), probably in relation to the different time point used for patient inclusion, as the revascularization rate is higher when recruitment is more recent. Furthermore, the rates of statin and beta-blocker therapy at study inclusion are low for current guidelines, but reflect routine practice at the time.
In our population, higher mortality was observed during long-term follow-up, associated with baseline variables such as DM2, smoking, resting HR, baseline AF, or age. Although active smokers and exsmokers had lower mortality in the univariate analysis, once an adjustment was performed for confounding factors in the multivariate analysis, active smoking was found to be an independent predictor of all-cause mortality. These associations have been confirmed by a number of earlier studies,3,16–20,24,25 but the relationship between them and higher all-cause mortality in long-term follow-up had not been confirmed in Spain.
Angina was shown to be an independent predictor of cardiovascular mortality, which confirms the results of previous studies, such as the CLARIFY registry.26 On the other hand, registries such as REACH have shown a weak relationship between angina and cardiovascular mortality.19
An abnormal baseline electrocardiogram was also significantly associated with higher all-cause and cardiovascular mortality during follow-up of this population. Previous studies found an independent association with a higher all-cause mortality even in the general population with abnormalities in the baseline electrocardiogram.27
However, cardiomegaly on chest radiography was associated with all-cause and cardiovascular mortality in the univariate analysis, which is relevant in the historic context of recruitment, when there was little access to cardiac imaging tests. Despite the undeniable association with heart failure, cardiomegaly is not reliably associated with LVEF and could be considered an independent marker of poor long-term prognosis, as reported by other studies.28
Last, mortality was somewhat influenced by certain medical therapies: those closely related to heart failure, such as angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists and diuretics, were associated with higher mortality during follow-up in the univariate analysis, whereas currently well-established drugs for the treatment of SIHD, such as statins and antiplatelets, were associated with lower mortality. Only diuretic therapy showed an independent relationship with mortality in additional analyses that included medical treatment variants, probably related to the presence by underlying heart failure.
It is worth noting that 5 of 6 independent predictors of cardiovascular and all-cause mortality are shared. This would seem logical if most deaths were of cardiovascular cause, but this was not the case in our study, although, as stated earlier, it cannot be ruled out with certainty that the proportion of cardiovascular deaths was actually higher than the proportion detected.
Limitations and StrengthsThis study has some limitations. Because it was a single-center study, the findings should be interpreted with caution even though the baseline characteristics are similar to those seen in SIHD registries in Spain. The long follow-up period makes it difficult to ensure that the medical treatments initially recorded were prescribed throughout follow-up. Data on LVEF should be taken with caution, as the rate of lost values was> 10%. Another limitation of the sample is that initial recruitment did not include variables that could influence prognosis, such as kidney function, prior hospitalizations due to heart failure, hemoglobin levels, or white blood cell counts. Despite the investigators’ efforts, 30% of deaths were of unknown cause, which could limit the reliability of the information obtained on cardiovascular mortality.
Last, when mortality rates are compared with those of the Spanish population, the available data only allow adjustment by age, sex, and year and do not consider other possibly relevant factors.
This study has several strengths. Most importantly, the study has the longest follow-up of a broad SIHD sample reported in Spain, thus allowing a large number of events that ensured sufficient statistical power for the analysis. Moreover, the events were verified and classified by the investigators, thus avoiding the uncertainties involved in analyzing administrative databases.
CONCLUSIONSIn this sample of patients with SIHD, obtained during routine clinical practice, the probability of 12-year survival was 56%, significantly lower than that of the Spanish population of similar age and sex distribution. Clinical variables could identify patients with a higher risk of mortality during follow-up.
CONFLICTS OF INTERESTNone declared.
- –
Improvements in the treatment of acute coronary syndrome have increased the prevalence of stable ischemic heart disease.
- –
The mortality rate of these patients was similar to that of the general population in previous studies carried out in other countries and in the placebo groups of clinical trials. These studies have allowed definition of variables associated with higher mortality.
- –
However, there are no contemporary observational studies investigating the very long-term mortality of patients with this disease in routine clinical practice in Spain.
- –
This study focused on the prognosis of stable ischemic heart disease with a longer follow-up period in our setting.
- –
The annual mortality rate of patients with stable ischemic heart disease was significantly higher than that of the general population.
- –
Certain baseline clinical variables could help us stratify mortality risk during patient follow-up.