A variety of cardiac magnetic resonance indexes predict mid-term prognosis in ST-segment elevation myocardial infarction patients. The extent of transmural necrosis permits simple and accurate prediction of systolic recovery. However, its long-term prognostic value beyond a comprehensive clinical and cardiac magnetic resonance evaluation is unknown. We hypothesized that a simple semiquantitative assessment of the extent of transmural necrosis is the best resonance index to predict long-term outcome soon after a first ST-segment elevation myocardial infarction.
MethodsOne week after a first ST-segment elevation myocardial infarction we carried out a comprehensive quantification of several resonance parameters in 206 consecutive patients. A semiquantitative assessment (altered number of segments in the 17-segment model) of edema, baseline and post-dobutamine wall motion abnormalities, first pass perfusion, microvascular obstruction, and the extent of transmural necrosis was also performed.
ResultsDuring follow-up (median 51 months), 29 patients suffered a major adverse cardiac event (8 cardiac deaths, 11 nonfatal myocardial infarctions, and 10 readmissions for heart failure). Major cardiac events were associated with more severely altered quantitative and semiquantitative resonance indexes. After a comprehensive multivariate adjustment, the extent of transmural necrosis was the only resonance index independently related to the major cardiac event rate (hazard ratio=1.34 [1.19-1.51] per each additional segment displaying>50% transmural necrosis, P<.001).
ConclusionsA simple and non-time consuming semiquantitative analysis of the extent of transmural necrosis is the most powerful cardiac magnetic resonance index to predict long-term outcome soon after a first ST-segment elevation myocardial infarction.
Keywords
Cardiac magnetic resonance (CMR) allows a simultaneous analysis of a variety of cardiac indexes, and it has become the state-of-the-art noninvasive technique for assessing the structural consequences of ST-segment elevation myocardial infarction (STEMI).1–4 Beyond its well-demonstrated diagnostic value, solid validation of the prognostic usefulness of this technique is a mandatory step before recommending a generalized use in routine practice.5
So far, several CMR indexes have already shown their short and mid-term prognostic value.6–15 Some caveats, though, are necessary to interpret these studies. First, all of them were limited to short- or mid-term follow-up. Second, most studies were focused on time-consuming indexes, highly relevant in terms of research but difficult to implement in busy CMR labs. Third, adjustment for not only traditional risk prognosticators, but also for the wide range of currently available quantitative and semiquantitative CMR indexes has not been performed yet. And last, but not least, a tendency towards highlighting the predictive utility of individual indexes has resulted in a plethora of CMR parameters which are suggested to independently predict patient outcomes soon after STEMI. The latter could induce certain confusion and discouragement among clinicians to transfer results derived from registries into clinical practice.
Kim et al.16 were the first to demonstrate that the extent of transmural necrosis (ETN) was the CMR index most strongly related to systolic recovery in chronic ischemic patients. We confirmed, soon after STEMI, that ETN was the CMR parameter most powerfully associated not only with systolic recovery but also with mid-term prognosis.1,10,15 The parallelism between late gadolinium enhancement CMR imaging and the extent of necrotic myocardium is the solid pathophysiological basis underlying these findings.
We hypothesized that a simple semiquantitative assessment of ETN based on the number of segments showing transmural necrosis is the best and most suitable CMR index to predict very long-term outcome soon after STEMI beyond a comprehensive adjustment for clinical, electrocardiographic, biochemical, angiographic, quantitative, and semiquantitative CMR variables.
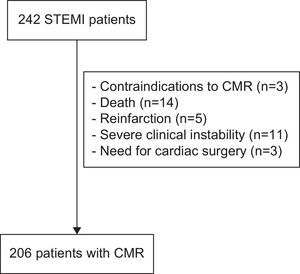
MATERIAL AND METHODSStudy GroupFrom January 2004 to December 2006, we prospectively included 242 consecutive patients admitted to a tertiary university hospital with a first STEMI. The exclusion criteria were: contraindications to CMR (n=3), death (n=14), reinfarction (n=5), severe clinical instability (n=11), and need for cardiac surgery (n=3) during admission. Accordingly, the study group comprised 206 patients without serious complications during admission (Fig. 1).
The local ethics committee approved the research protocol. Written informed consent was obtained from all subjects.
Reperfusion TherapyReperfusion strategy and medical treatment were left to the discretion of the attending cardiologists.
Primary angioplasty was performed in 51 (25%) patients. A pharmaco-invasive strategy was carried out in 122 patients (59%): thrombolytic agents were administered and if reperfusion criteria were achieved (100 patients), elective catheterization and angioplasty if needed were performed within the first 24h. Rescue angioplasty was performed in 22 patients due to ineffective reperfusion after thrombolytic therapy.
Thirty-four patients (17%) were not treated with any reperfusion strategy within the first 12h due to delayed presentation; in all of them, elective catheterization and angioplasty if needed were performed within the first 24h (Table 1).
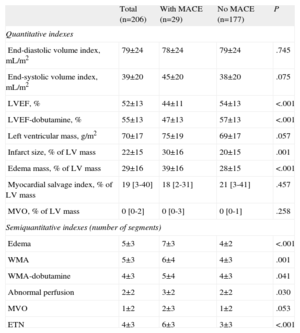
Clinical, Electrocardiographic, Biochemical and Angiographic Characteristics for the Entire Study Group and for Patients With and Without Major Adverse Cardiac Events
| Total (n=206) | With MACE (n=29) | No MACE (n=177) | P | |
| Age, years | 58±12 | 61±11 | 57±12 | .127 |
| Male | 175 (85) | 25 (86) | 150 (85) | 1.000 |
| Smoker | 129 (63) | 18 (62) | 111 (63) | 1.000 |
| Hypertension | 85 (41) | 13 (45) | 72 (41) | .689 |
| Hypercholesterolemia | 73 (35) | 11 (38) | 62 (35) | .835 |
| Diabetes | 30 (15) | 4 (14) | 26 (15) | 1.000 |
| Ischemic cardiac disease | 9 (4) | 4 (14) | 5 (3) | .024 |
| Anterior infarction | 117 (57) | 23 (79) | 94 (53) | .009 |
| Heart rate, bpm | 81±21 | 90±29 | 79±19 | .007 |
| Systolic blood pressure, mmHg | 125±24 | 130±30 | 124±23 | .215 |
| Killip 1 | 189 (92) | 28 (96) | 161 (91) | .477 |
| Killip 2 | 16 (8) | 1 (3) | 15 (8) | .706 |
| Killip 3 | 1 | 0 | 1 | 1.000 |
| TIMI risk score | 2±2 | 3±2 | 2±2 | .021 |
| Early reperfusion therapy | 172 (83) | 26 (90) | 146 (82) | .427 |
| Time to reperfusion, min | 237±175 | 256±177 | 234±175 | .553 |
| Primary angioplasty | 51 (25) | 7 (24) | 44 (25) | 1.000 |
| Thrombolysis | 122 (59) | 19 (65) | 116 (58) | .543 |
| Rescue angioplasty | 22 (11) | 5 (17) | 17 (10) | .207 |
| Anti-GPIIb/IIIa | 84 (41) | 9 (31) | 75 (42) | .310 |
| Peak CK-MB mass, ng/mL | 193 [87-330] | 277 [111-500] | 187 [86-302] | .057 |
| Peak Troponin I, ng/mL | 60±41 | 68±34 | 65±42 | .693 |
| Glucose, mg/dL | 129±51 | 138±45 | 128±52 | .335 |
| Creatinine, mg/dL | 0.96±0.23 | 1.06±0.3 | 0.95±0.22 | .017 |
| Peak leukocyte count, ×109 cells/mL | 13.8±3.8 | 13.7±4.4 | 13.9±3.7 | .877 |
| Maximum residual ST elevation, mm | 10±7 | 14±9 | 10±7 | .001 |
| Number of Q-waves | 2±1 | 3±1 | 2±1 | .085 |
| TIMI III pre-stent | 100 (49) | 16 (55) | 84 (47) | .548 |
| TIMI III post-stent | 197 (96) | 28 (97) | 169 (95) | 1.000 |
| Blush 2-3 post-stent | 157 (76) | 18 (62) | 139 (79) | .062 |
| Multivessel | 32 (16) | 7 (24) | 25 (14) | .173 |
| Proximal LAD | 52 (25) | 11 (38) | 41 (23) | .107 |
| Stent | 180 (87) | 23 (79) | 157 (89) | .222 |
CK-MB, MB fraction of creatine kinase; GPIIb/IIIa, glucoprotein IIb/IIIa; LAD, left anterior descending coronary artery; MACE, major cardiovascular events; TIMI, Thrombolysis In Myocardial Infarction.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
Thrombolysis In Myocardial Infarction (TIMI) flow grade and myocardial blush grade were determined offline by an experienced observer unaware of CMR results using the software Integris HM3000 (Philips; Best, the Netherlands). TIMI flow grade 3 and myocardial blush grade 2 to 3 were regarded as normal.17
Clinical, Biochemical, and Electrocardiographic CharacteristicsBaseline characteristics were recorded. TIMI risk score for STEMI was calculated in all patients as a surrogate of baseline clinical risk.18 Peak troponin I, peak leukocyte, and glucose and creatinine upon admission were assessed. The number of Q waves and the maximum residual ST-segment elevation (considered as the sum of ST-segment elevation after reperfusion in leads V1–V6, I, and aVL for anterior infarction and in II, III, aVF, V5, and V6 for nonanterior infarction) were determined.
Cardiac Magnetic ResonanceAt 7 (2) days after STEMI, CMR (1.5-T scanner, Sonata Magnetom, Siemens; Erlangen, Germany) was performed in accordance with our laboratory protocol,2,10,15,19,20 as described in supplementary material.
An experienced observer blinded to all patient data analyzed CMR studies offline with customized software (QMASS MR 6.1.5, Medis; Leiden, the Netherlands). CMR indexes were determined both quantitatively (as defined below) and semiquantitatively (using the number of altered segments in the 17-segment model).21 The cutoff values used to consider extensive abnormalities were established on the basis of the receiver-operating characteristics curves for predicting major adverse cardiac events (MACE).
EdemaWe quantitatively determined, by manual definition, the percentage of left ventricular (LV) mass showing edema as a myocardial area having a signal intensity>2 standard deviations above the signal from remote noninfarcted myocardium in T2-weighted sequences. Semiquantitatively, we assessed the number of segments with edema. Extensive edema was considered if it was detected in >6 segments.
Wall Motion AbnormalitiesWe quantitatively determined the end-diastolic and end-systolic volume indexes (mL/m2), LV indexed mass (g/m2), and LV ejection fraction (LVEF, %). We defined systolic dysfunction according to LVEF established cutoffs for sex, body surface area, and age.22 Semiquantitatively, we recorded the number of segments with wall motion abnormalities (WMA): hypokinesia, akinesia, or dyskinesia. Extensive WMA was considered if present in >6 segments.
Wall Motion Abnormalities-dobutamineWe quantitatively determined LVEF during low-dose dobutamine infusion (LVEF-dobutamine, %). Semiquantitatively, we assessed the number of segments with WMA during low-dose dobutamine infusion. Extensive WMA-dobutamine was considered if present in >6 segments.
Abnormal First Pass PerfusionThis index was qualitatively defined as myocardium showing hypoenhancement at the end of the acquisition period in first-pass perfusion imaging. We semiquantitatively considered the number of segments with abnormal first pass perfusion. Extensive abnormal first pass perfusion was considered if it was detected in >2 segments.
Microvascular ObstructionWe quantitatively determined the percentage of LV mass showing microvascular obstruction, defined as lack of contrast uptake in the core of a segment surrounded by tissue showing late gadolinium enhancement. Semiquantitatively, we considered the number of segments with microvascular obstruction. Extensive microvascular obstruction was considered if it was detected in >4 segments.
Extent of Transmural NecrosisTransmural necrosis was considered when >50% of the thickness of the myocardial wall showed late gadolinium enhancement (defined as a signal intensity>2 standard deviations above the signal from remote noninfarcted myocardium). We quantitatively determined infarct size by manual definition as the percentage of LV mass showing late gadolinium enhancement and myocardial salvage index as the percentage of LV mass with edema not presenting late gadolinium enhancement. Semiquantitatively, we considered ETN as the number of segments with transmural necrosis in the 17-segment model. Extensive transmural necrosis was considered if it was detected in >6 segments.
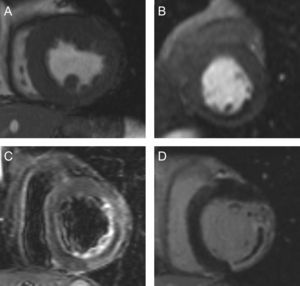
Figure 2 illustrates the variety of quantitative and semiquantitative CMR indexes determined. Intraobserver variability in our laboratory has been previously analyzed and is less than 5% for all indexes15.
Quantitative and semiquantitative indexes assessed in the present study. A: Cine images: end-diastolic and end-systolic volume indexes (mL/m2); left ventricular mass (g/m2); left ventricular ejection fraction (%); left ventricular ejection fraction-dobutamine (%); wall thickening (mm); wall motion abnormalities (segments); wall motion abnormalities-dobutamine (segments). B: First pass perfusion: abnormal first pass perfussion (segments). C: T2-weighted images: edema (% of left ventricular mass); edema (segments). D: Late enhancement images: infarct size (%); microvascular obstruction (% of left ventricular mass); transmural necrosis (segments); microvascular obstruction (segments); myocardial salvage index (%).
The end-point was MACE, defined as cardiac death, admission for nonfatal myocardial infarction, and admission for heart failure, whichever occurred first. Cardiac death was defined as death due to acute myocardial infarction, congestive heart failure, arrhythmias or unexpected sudden cardiac arrest. Myocardial infarction23 and heart failure24 were defined following current definitions.
Follow-up was performed by 2 cardiologists and 2 trained nurses from at least one of 3 sources: a) outpatient clinics; b) telephone interview with the patient or his/her family, or c) review of patient's hospital record. Consensus between both cardiologists was required to finally adjudicate an event.
Statistical AnalysisThe Kolmogorov-Smirnov test was used to evaluate the normal distribution of continuous data. Normally distributed variables were expressed as mean (standard deviation) and compared with the unpaired Student t test. Those variables without a normal distribution were expressed as median [interquartile range] and compared with the Mann-Whitney U test. Dichotomous variables were expressed as percentages and compared with the chi-square statistic or the Fisher's exact test when appropriate. Survival distributions for the time to event were estimated with the Kaplan-Meier method and the log rank test.
The association of variables with MACE was assessed with multivariate Cox proportional hazard regression models. The multivariate models to predict MACE were adjusted for variables identified by comparing patients who exhibited MACE during follow-up to those who did not; variables with P<.2 in univariate analyses were included in the regression models as cofactors. Hazard ratios (HR) with corresponding 95% confidence intervals (95%CI) were computed.
In order to evaluate the additional prognostic value of CMR indexes beyond traditional risk stratification, we determined the C-statistic and the −2 log likelihood of a multivariate model including clinical, electrocardiographic, biochemical, and angiographic variables, along with CMR-derived LVEF (which, in general, is available in all STEMI patients using echocardiography). Then we determined the C-statistic and the −2 log likelihood of the final multivariate model, which also included all CMR indexes. We also explored the prognostic effect of ETN based on the analysis of quartiles. The risk of MACE along the continuum of the number of segments with transmural necrosis was evaluated in the context of multivariate fractional polynomials (4 degrees of freedom).
A two-tailed P value<.05 was considered to indicate a statistically significant difference. The SPSS version 13.0 (SPSS Inc.; Chicago, Illinois, United States) software and STATA 11.1 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, Texas: StataCorp LP, United States) were used.
RESULTSDuring a median follow-up of 51 months (range 40 to 74), 39 MACE were detected in 29 patients including 12 cardiac deaths, 15 nonfatal myocardial infarctions, and 12 readmissions for heart failure. The first event in those 29 patients in whom MACE took place was death in 8 patients, nonfatal myocardial infarction in 11, and readmission for heart failure in 10.
Patients with MACE presented a higher rate of previous ischemic cardiomyopathy, higher heart rate at admission, anterior myocardial infarction, worse TIMI risk score-derived clinical profile, more residual ST-segment elevation, and higher creatinine level. Clinical, electrocardiographic, biochemical, and cardiac catheterization variables of the whole study group and of patients with and without MACE are displayed in Table 1.
Major Cardiovascular Events and Cardiac Magnetic Resonance Data. Univariate AnalysisThe CMR characteristics of the whole study group and of patients with and without MACE are displayed in Table 2.
Cardiac Magnetic Resonance Characteristics for the Entire Study Group and for Patients With and Without Major Adverse Cardiac Events
| Total (n=206) | With MACE (n=29) | No MACE (n=177) | P | |
| Quantitative indexes | ||||
| End-diastolic volume index, mL/m2 | 79±24 | 78±24 | 79±24 | .745 |
| End-systolic volume index, mL/m2 | 39±20 | 45±20 | 38±20 | .075 |
| LVEF, % | 52±13 | 44±11 | 54±13 | <.001 |
| LVEF-dobutamine, % | 55±13 | 47±13 | 57±13 | <.001 |
| Left ventricular mass, g/m2 | 70±17 | 75±19 | 69±17 | .057 |
| Infarct size, % of LV mass | 22±15 | 30±16 | 20±15 | .001 |
| Edema mass, % of LV mass | 29±16 | 39±16 | 28±15 | <.001 |
| Myocardial salvage index, % of LV mass | 19 [3-40] | 18 [2-31] | 21 [3-41] | .457 |
| MVO, % of LV mass | 0 [0-2] | 0 [0-3] | 0 [0-1] | .258 |
| Semiquantitative indexes (number of segments) | ||||
| Edema | 5±3 | 7±3 | 4±2 | <.001 |
| WMA | 5±3 | 6±4 | 4±3 | .001 |
| WMA-dobutamine | 4±3 | 5±4 | 4±3 | .041 |
| Abnormal perfusion | 2±2 | 3±2 | 2±2 | .030 |
| MVO | 1±2 | 2±3 | 1±2 | .053 |
| ETN | 4±3 | 6±3 | 3±3 | <.001 |
ETN, extent of transmural necrosis; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major cardiovascular events; MVO, microvascular obstruction; WMA, wall motion abnormalities.
Data are expressed as mean±standard deviation or median [interquartile range].
Regarding quantitative CMR indexes, patients with MACE showed more depressed LVEF, lower LVEF-dobutamine, larger infarct size, larger percentage of LV mass with edema, and a trend towards larger end-systolic volume index and more LV mass (Table 2).
With respect to semiquantitative CMR indexes, patients with MACE displayed a larger extent (number of segments) of the six CMR indexes evaluated: edema, WMA, WMA-dobutamine, abnormal first pass perfusion, microvascular obstruction, and transmural necrosis (Table 2).
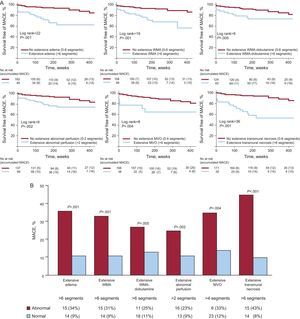
Extensive abnormalities in semiquantitative indexes were detected as follows: edema in 44 patients (21%), WMA in 48 (23%), WMA-dobutamine in 44 (21%), abnormal first pass perfusion in 69 (33%), microvascular obstruction in 18 (9%), and ETN in 35 (17%). The presence of extensive abnormalities in all CMR indexes evaluated were significantly related to higher probability of MACE during follow-up (P<.01 for all comparisons, Fig. 3).
A: Kaplan-Meier curves of patients with and without extensive abnormalities of the semiquantitative indexes evaluated. B: major adverse cardiovascular events rates of patients with and without extensive abnormalities of the semiquantitative indexes evaluated. MACE: major adverse cardiovascular events; MVO, microvascular obstruction; WMA, wall motion abnormalities.
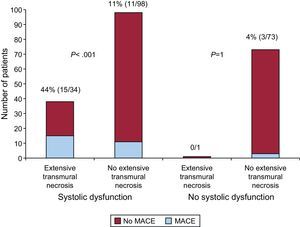
The MACE rate was 20% in patients with systolic dysfunction and 4% in patients without systolic dysfunction (P=.001). Among patients with systolic dysfunction, patients with ETN had a higher rate of MACE than patients without ETN (44% vs 11%, P<.001). To the contrary, among patients without systolic dysfunction, MACE rate was not different between the subgroups of patients with and without ETN (0% vs 4%, P=1). This is also illustrated in Figure 4.
Number of patients with and without major adverse cardiovascular events according to the presence of systolic dysfunction and extensive transmural necrosis. In patients without systolic dysfunction, unlike in the group of patients with systolic dysfunction, major adverse cardiovascular events were rare and not related to the presence of extensive transmural necrosis. Systolic dysfunction was defined according to the established cut-off points for age, gender and body surface. MACE, major adverse cardiovascular event.
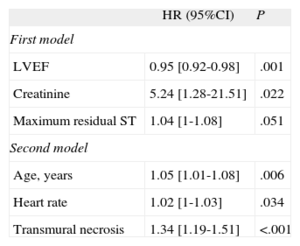
Variables with P-value<.2 in Table 1 along with CMR-derived LVEF were tested in a first multivariate analysis, thus including data conventionally used in long-term post-STEMI risk stratification. In this approach, variables independently associated with MACE were LVEF, creatinine levels, and maximum residual ST segment elevation (Table 3). C-statistic of the resulting model was 0.75 [0.66-0.85].
Multivariate Analysis
| HR (95%CI) | P | |
| First model | ||
| LVEF | 0.95 [0.92-0.98] | .001 |
| Creatinine | 5.24 [1.28-21.51] | .022 |
| Maximum residual ST | 1.04 [1-1.08] | .051 |
| Second model | ||
| Age, years | 1.05 [1.01-1.08] | .006 |
| Heart rate | 1.02 [1-1.03] | .034 |
| Transmural necrosis | 1.34 [1.19-1.51] | <.001 |
95%CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
First model included age, anterior infarction, heart rate, Thrombolysis In Myocardial Infarction risk score, creatinine, peak MB fraction of creatine kinase mass, maximum residual ST segment elevation, number of Q-waves, blush 2-3 post-stent, proximal left anterior descending coronary artery disease, and LVEF. C-statistic of the resulting model was 0.75 [0.66-0.85]. Second model included the same variables as the first model and all cardiac magnetic resonance indexes determined. The C-statistic of the final model was 0.83 [0.75-0.91]. The −2 log likelihood statistic was significantly lower in the second model (258.3 vs 268; P<.005).
In a second comprehensive multivariate analysis, we included all variables tested in the first model as well as those CMR parameters showing p-value<0.2. Along with more advanced age and higher heart rate at admission, the semiquantitative assessment of ETN was the only CMR index independently associated with MACE (HR=1.34; 95%CI, 1.19-1.51; P<.001) (Table 3). The C-statistic of the final model was 0.83 [0.75–0.91]. The −2 log likelihood statistic was significantly lower in the second model (258.3 vs 268; P<.005), suggesting that ETN yielded additional very long-term prognostic information beyond the traditional prognostic variables.
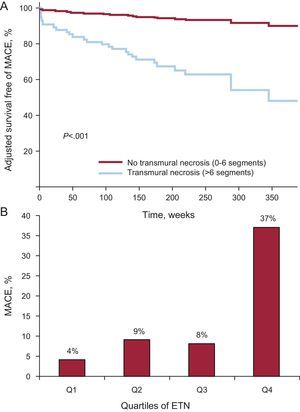
Therefore, the semiquantitative assessment of ETN appeared as the variable most powerfully associated with very long-term post-STEMI MACE. Adjusted survival curves displayed striking differences in the time to first MACE when comparing patients with and without ETN (Fig. 5). A steady increase in MACE rate was observed when ETN was categorized in quartiles (number of segments having >50% transmural necrosis) (Fig. 5).
A: Adjusted survival free of major adverse cardiovascular events in patients with and without extensive transmural necrosis. B: major adverse cardiovascular events rate according to quartiles of extension of transmural necrosis: Q1, <3 segments; Q2, 3 segments; Q3, 4-5 segments, and Q4, >6 segments. ETN, extension of transmural necrosis; MACE, adverse cardiovascular events.
When analyzing the risk of MACE along the continuum of the number of segments with transmural necrosis, we found that the risk attributable to ETN showed a linear gradient (Figure in supplementary material).
DISCUSSIONThe main finding of the present study is that a simple, non-time-consuming and intuitive index based on a semiquantitative assessment of ETN independently predicts very long-term STEMI patients’ outcome beyond a comprehensive adjustment for a wide range of clinical, electrocardiographic, biochemical, and angiographic prognosticators, as well as for a variety of quantitative and semiquantitative CMR indexes.
The follow-up (median>4 years) is by far the longest reported. We undertook a comprehensive CMR evaluation which included a wide range of time-consuming quantitative indexes along with simple and intuitive semiquantitative parameters based on the number of segments displaying abnormalities; of note, we included low-dobutamine response in our analysis, a solid predictor of systolic recovery in STEMI patients which has not been taken into account in the majority of previous studies. Adjustment for baseline and TIMI score-derived clinical variables, electrocardiographic, biochemical, and angiographic variables was also carried out.
Previous StudiesUndoubtedly, systolic function represents a major predictor of clinical outcome after infarction. In accordance with existing knowledge, patients with systolic dysfunction in our study had a higher MACE rate. In revascularized patients LVEF may present dramatic changes in the months following STEMI, which may have striking influence on patient outcomes. Thus, the rationale of using additional imaging techniques such as CMR is based not only on identifying indexes that reliably predict systolic recovery, but also on scrutinizing the prognostic value of these indexes.
In parallel to recently published studies, our data confirm that, separately, all quantitative and semiquantitative CMR indexes evaluated contribute prognostic information after STEMI.
The presence of myocardial edema offers unique information regarding the extent of area at risk during coronary occlusion. It has been recently incorporated into our armamentarium and can only be evaluated by means of CMR.19,20 Data on the prognostic value of edema in patients with STEMI are scarce. By comparing area at risk and the final infarct size, Eitel et al.11 have reported that a larger myocardial salvage index is associated with better prognosis in STEMI patients. In our study, a greater extent of edema was associated with worse prognosis, but the myocardial salvage index was not. What may underlie the low predictive value of this index is the fact that some patients display a huge area at risk and, despite a large myocardial salvage index, the resulting infarct can still be large.
The usefulness of WMA-dobutamine to predict systolic recovery and prognosis has been solidly addressed using stress-echocardiography. Though the excellent spatial resolution of CMR permits an accurate evaluation of contractile reserve, a prolongation of studies along with the need for inotropic drugs in a poorly monitored environment creates some drawbacks. Although in univariate analysis a lesser extent of contractile reserve predicted a higher MACE rate, the before-mentioned difficulties, along with the fact that ETN overcomes the predictive WMA-dobutamine information, clearly suggest limiting the use of this approach in selected cases.
CMR is able to study microvascular perfusion,1,2,6,7,10,12,25 either using first-pass perfusion or late gadolinium enhancement sequences. Wu et al.6, in a small group of 44 patients, demonstrated the prognostic value of an intermediate step 1-2min after contrast infusion. De Waha et al.12 suggested that microvascular obstruction (as derived from late gadolinium enhancement imaging) is an independent prognostic factor after STEMI. Similar results were obtained by Hombach et al.7 in a heterogeneous group including STEMI and non-STEMI patients. In univariate analyses our results confirm the association between abnormal microvascular perfusion and higher MACE rate, but this index is not independently associated with prognosis in multivariate analysis, similar to other studies.11,14 This could be explained in part because some cases with microvascular obstruction display a small infarcted area with little prognostic significance (for example in the case of the delayed reperfusion of a marginal branch). Additionally, this illustrates the predictive power of the simple approach used in this study to quantify ETN, which simultaneously contemplates the extension and transmurality of the infarcted area.
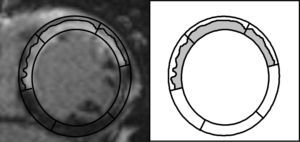
Implications of the Present StudyFrom a very long-term perspective and after comprehensive adjustment, not only for traditional prognosticators but also for the wide variety of CMR indexes currently available, we confirmed that a semiquantitative assessment of ETN represents the CMR parameter most robustly associated with prognosis in STEMI patients. From a pathophysiological point of view, this finding could probably rely on the fact that this index embraces at a glance relevant information regarding: a) transmurality (all segments included in this index display >50% of transmural necrosis), and b) extent of necrosis (a larger number of altered segments obviously suggests more extensive transmural infarction) (Fig. 6).
From a practical point of view, these results portend clear advantages. The semiquantitative assessment of ETN can be rapidly and accurately interpreted by experienced observers. Even after adjustment for a variety of time-consuming CMR variables, this simple index appeared as the most robust CMR prognosticator. Thus, on the basis of: a) the solid pathophysiological association with the extent of myocardial necrosis; b) well-proven usefulness to predict late systolic recovery; c) very-long term prognostic value, and last but not least, d) its simplicity, ETN can become a workhorse in the CMR evaluation of post-STEMI patients. In this scenario, therefore, simpler is better. The cut-off point of ETN>6 segments indicates that around 30% of the mass of LV seems the best cut-off point for predicting MACE. We had previously reported that a similar cut-off point (>5 segments) best predicted outcome in a mid-term follow-up.10
However, in an era of budget shortage, CMR may not be available for all patients. Our results suggest that, for very long-term prognostic purposes, LVEF represents a simple tool that permits identifying those patients who benefit most from a CMR study soon after STEMI. Whereas, in general, revascularized patients with preserved LVEF have an excellent very long-term prognosis (4% MACE rate in our series), outcome of those cases with depressed LVEF is variable. According to our results, the latter represents the subset of patients in whom CMR soon after STEMI can more efficiently prognosticate very long-term outcome: in patients with depressed LVEF MACE rate was 4-fold higher (44% vs 11%) in those cases with extensive ETN.
Study LimitationsAlthough this study represents the largest series of patients with a long-term follow-up, the limited number of events explains the small variations in the best cut-off values in CMR parameters to predict events with respect to our previous data analyzing short- and mid-term follow-up.10 This also explains why, in addition to the powerful prognostic significance of ETN, the independent predictors of MACE vary depending on the variables included in the multivariate analysis. The almost universal use of pre-discharge percutaneous revascularization along with the exclusion of cases with in-hospital events or severe hemodynamic instability may explain this low MACE rate. The future therapeutic opportunities derived from our results need further studies and to address larger study groups.
CONCLUSIONSA simple and non-time-consuming semiquantitative analysis of ETN is the CMR index most strongly associated with very long-term outcome soon after a first STEMI even after adjustment for a wide variety of clinical, electrocardiographic, biochemical, angiographic, quantitative, and semiquantitative CMR parameters. Patients with depressed LVEF represent the subset of cases that, for prognostic purposes, benefit most from the analysis of this parameter.
FUNDINGThis study was funded with grant FIS PI11/02323, Microcluster Protección Cardiovascular VLC-Campus of Excellence, Fundació Gent per Gent, Ayuda para Grupos Emergentes INCLIVA.
CONFLICTS OF INTERESTNone declared.