Many patients with nonvalvular atrial fibrillation are still left without protection due to a contraindication for anticoagulants. This study aimed to establish the occurrence of stroke and major bleeding events in patients with nonvalvular atrial fibrillation and left atrial appendage closure with long-term follow-up and to explore the factors associated with higher long-term mortality.
MethodsAnalysis of a multicenter single cohort prospectively recruited from 2009 to 2015. Thromboembolic and bleeding events were compared with those expected from CHA2DS2-VASc and HAS-BLED scores. Multivariate analysis examined variables associated with mortality during follow-up.
ResultsA total of 598 patients (1093 patient-years) with a contraindication for anticoagulants were recruited (median 75.4 years). The success rate of left atrial appendage closure device implantation was 95.8%. Thirty patients (5%) experienced periprocedural complications. The rate of events (per 100 patient-years) during follow-up (mean 22.9 months; median 16.1 months) was as follows: death 7.0%; ischemic stroke 1.6% (vs 8.5% expected according to CHA2DS2-VASc; P < .001); intracranial hemorrhage 0.8%; gastrointestinal bleeding 3.2%; severe bleeding 3.9% (vs 6.3% expected by HAS-BLED, P = .002). These results were improved in the subgroup of 176 patients with follow-up > 24 months (mean follow-up 46.6 months, 683 patient-years) for severe bleeding 2.6% (vs 6.3% expected by HAS-BLED, P < .033). The factors significantly associated with higher mortality were age (HR, 1.1), intracranial hemorrhage (HR, 6.8), and stroke during follow-up (HR, 2.7).
ConclusionsLeft atrial appendage closure significantly reduced the incidence of stroke and bleeding events and the benefit was maintained. Intracranial hemorrhage, age and stroke were associated with higher mortality.
Keywords
Nonvalvular atrial fibrillation is a major health problem, particularly in the older population.1 One of the main positive aspects of the new oral anticoagulants (NOAC) is that a larger number of patients at risk of stroke are now receiving treatment.2 Nevertheless, this increase has been slow, and even lower than expected in some registries.3,4 Thus, there are still a considerable number of patients who, due to high bleeding risk, previous history of bleeding while on NOAC treatment, or lack of treatment adherence, are left without anticoagulant protection.5,6 In the GARFIELD-AF study,5 despite including populations with a mean HAS-BLED (hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score of 1, at least 30% of patients who should have been on NOAC did not receive this treatment. These percentages were higher as the HAS-BLED score increased, perhaps reflecting patients’ and clinicians’ fear of using these treatments in patients with a history of previous bleeding or high bleeding risk scores.
Closure of the left atrial appendage (LAA) is a strategy that is useful for treating these types of patients.7 Both randomized studies in patients who were able to take oral anticoagulants (OAC) and registries of those with a contraindication for anticoagulants have shown a reduction in mortality and thromboembolic/bleeding events with LAA closure.8–11 Although guidelines put LAA closure in a class IIb indication in this context, this recommendation is not shared by other investigators when it refers to patients with a contraindication for OAC, or in clinical practice by physicians who treat patients with different types of contraindications for OAC in real life.12–14
As follow-up in patients implanted with an LAA closure device becomes longer, we will achieve a better understanding of their natural history. The main aim of this study was, therefore, to examine the occurrence of thromboembolic and bleeding events in a long-term follow-up of 2 years (or beyond 2 years in a subgroup of patients), as well as the main predictors for long-term mortality.
METHODSDesign, Patients, and ProceduresA total of 598 patients from 13 tertiary referral hospitals across the Iberian Peninsula (10 from Spain, and 3 from Portugal) who underwent LAA closure between March 2, 2009 and December 18, 2015 were prospectively reviewed. These were the set of patients prospectively included in the Iberian Registry who are continuing long-term follow-up,15 plus additional patients successively included up to the end of the date set for end of recruitment. The devices used were the Amplatzer Cardiac Plug, Amplatzer Amulet, and Watchman. Follow-up was carried out by review of the scheduled control echocardiograms, and by means of outpatient consultations and/or a telephone call after the initial period.
Thromboembolic and bleeding events were compared with those expected from CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke history, vascular disease, sex) and HAS-BLED scores in the overall sample and in patients with > 24-months’ follow-up.
All patients signed consent forms for the intervention and follow-up. The study protocol was approved by the hospital ethics committee.
All patients underwent transesophageal echocardiography (TEE) within 24-48hours before the procedure or at least within the previous week to rule out the presence of LAA thrombus. Subsequent antithrombotic treatment consisted of a loading dose (600mg) of clopidogrel after the implantation, and initiation of treatment with 300mg of aspirin on the first day and 100mg daily thereafter. Clopidogrel was maintained for 3 to 6 months, except in the event of onset of bleeding complications, and aspirin for at least 6 to 12 months.
Very strict clinical follow-up with TEE was performed in at least 2 time periods between 1 to 3 months and 3 to 6 months. In the event of a thrombus, subcutaneous enoxaparin was added at therapeutic doses for 2 weeks, and the TEE was repeated to confirm that it had disappeared. If it persisted, we assessed whether to prolong treatment for another week, or to admit the patient and start intravenous heparin treatment.
Variables and DefinitionsThromboembolic EventsStroke was defined as an acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury, as a result of hemorrhage or infarction. A transient ischemic attack was distinguished from ischemic stroke, based on focal neurological symptoms lasting < 24hours and imaging-confirmed absence of acute brain infarction. Systemic embolism was defined as acute vascular insufficiency or occlusion of the extremities or of any organ outside the central nervous system that was associated with clinical, or other data of arterial occlusion.
Bleeding EventsMajor bleeding was defined as clinically overt bleeding, associated with any of the following: fatal outcome; involvement of a critical anatomic site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, or intramuscular with compartment syndrome); fall in hemoglobin concentration > 3g/dL; transfusion of > 2 units of whole blood or packed red blood cells; and need for hospital admission.
Risk EstimationThe baseline embolic risk profile was calculated using the CHADS2 (congestive heart failure, hypertension, age, diabetes, stroke history) and CHA2DS2-VASc scores, performing separate analyses with the Lip et al.16 and Friberg et al.17 series. Bleeding risk was calculated using the HAS-BLED score. Clinical events (especially total and cardiac mortality) and thrombotic and bleeding events requiring admission were evaluated at each visit. The observed incidence of events was calculated per patient and year of follow-up (number of patients at the start of the follow-up period, multiplied by the mean patient follow-up time expressed in years). The expected incidence of events in the sample was calculated as the mean individual risk of each patient.
In the TEE, the presence of a thrombus in the device, peridevice leak, presence of residual interatrial communication and confirmation that the device remained in the correct position were recorded. A thrombus was defined as the presence of an echocardiographic density visible in more than 1 plane, which was pedunculated and/or did not correspond to the usual laminar re-reendothelialization of the coating of the device. Identification was made by consensus between 2 specialists in echocardiograms. The presence of thrombus in the device was not considered an event unless a clinical thromboembolic event followed. A leak was interpreted as persistence of flow > 1mm through the edge of the device, with passage into the LAA.
Statistical AnalysisQuantitative variables are expressed as mean ± standard deviation or median (25th-75th percentile). Categorical variables are expressed as absolute frequency and percentage. Categorical variables were compared using the chi-square test or Fisher exact test, and quantitative variables using the Student t test or Wilcoxon test. Comparisons between rates of observed and expected events were evaluated using binomial tests. Event-free survival analysis was performed using the Kaplan-Meier method and Cox regression. Multivariate analysis was performed to determine clinical events that occurred during follow-up and which might be associated with higher mortality. Significance level was set at P < .05. All analyses were carried out using the SPSS statistical package, version 19. 0.
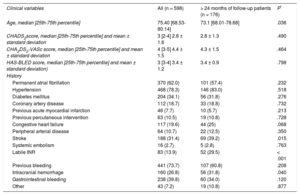
RESULTSThe baseline characteristics of the global population and the subgroup with the longest follow-up are shown in Table 1 (P refers to the difference with the group with < 24 months of follow-up). Median patient age was 75.4 years. Permanent atrial fibrillation was seen in 62% of patients and paroxysmal atrial fibrillation in 38%; 31.4% had a history of stroke, and 73.7% had a history of major bleeding. The CHA2DS2-VASc and HAS-BLED scores were 4.4 ± 1.5 and 3.4 ± 1.2, respectively. The devices used for LAA closure were the Amplatzer Cardiac Plug (n = 278; 46.5%), Amplatzer Amulet (n = 209; 34.9%), and Watchman (n = 111; 18.6%). Implantation was successful in 95.8% of cases: 93.9% for the Amplatzer Cardiac Plug, 98.1% for Amulet, and 96.4% for Watchman.
Population Baseline Variables
| Clinical variables | All (n = 598) | > 24 months of follow-up patients (n = 176) | P* |
|---|---|---|---|
| Age, median [25th-75th percentile] | 75.40 [68.53-80.14] | 73.1 [68.01-78.68] | .036 |
| CHADS2score, median [25th-75th percentile] and mean ± standard deviation | 3 [2-4] 2.8 ± 1.6 | 2.8 ± 1.3 | .490 |
| CHA2DS2-VASc score, median [25th-75th percentile] and mean ± standard deviation | 4 [3-5] 4.4 ± 1.5 | 4.3 ± 1.5 | .464 |
| HAS-BLED score, median [25th-75th percentile] and mean ± standard deviation) | 3 [3-4] 3.4 ± 1.2 | 3.4 ± 0.9 | .798 |
| History | |||
| Permanent atrial fibrillation | 370 (62.0) | 101 (57.4) | .232 |
| Hypertension | 468 (78.3) | 146 (83.0) | .518 |
| Diabetes mellitus | 204 (34.1) | 56 (31.8) | .276 |
| Coronary artery disease | 112 (18.7) | 33 (18.8) | .732 |
| Previous acute myocardial infarction | 46 (7.7) | 10 (5.7) | .213 |
| Previous percutaneous intervention | 63 (10.5) | 19 (10.8) | .728 |
| Congestive heart failure | 117 (19.6) | 44 (25) | .068 |
| Peripheral arterial disease | 64 (10.7) | 22 (12.5) | .350 |
| Stroke | 188 (31.4) | 69 (39.2) | .015 |
| Systemic embolism | 16 (2.7) | 5 (2.8) | .763 |
| Labile INR | 83 (13.9) | 52 (29.5) | < .001 |
| Previous bleeding | 441 (73.7) | 107 (60.8) | .208 |
| Intracranial hemorrhage | 160 (26.8) | 56 (31.8) | .040 |
| Gastrointestinal bleeding | 238 (39.8) | 60 (34.0) | .120 |
| Other | 43 (7.2) | 19 (10.8) | .877 |
Unless otherwise indicated, data are expressed as No. (%).
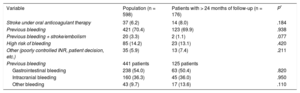
Indications for the procedure are shown in Table 2. The data from patients with follow-up > 24 months are described in Table 1. The high percentage of patients with a previous history of bleeding, particularly gastrointestinal bleeding, was notable.
Indications for the Procedure
| Variable | Population (n = 598) | Patients with > 24 months of follow-up (n = 176) | P* |
|---|---|---|---|
| Stroke under oral anticoagulant therapy | 37 (6.2) | 14 (8.0) | .184 |
| Previous bleeding | 421 (70.4) | 123 (69.9) | .938 |
| Previous bleeding + stroke/embolism | 20 (3.3) | 2 (1.1) | .077 |
| High risk of bleeding | 85 (14.2) | 23 (13.1) | .420 |
| Other (poorly controlled INR, patient decision, etc.) | 35 (5.9) | 13 (7.4) | .211 |
| Previous bleeding | 441 patients | 125 patients | |
| Gastrointestinal bleeding | 238 (54.0) | 63 (50.4) | .820 |
| Intracranial bleeding | 160 (36.3) | 45 (36.0) | .950 |
| Other bleeding | 43 (9.7) | 17 (13.6) | .110 |
Unless otherwise indicated, data are expressed as No. (%).
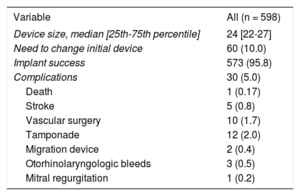
The main complications are shown in Table 3. Thirty patients (5%) experienced periprocedural complications, of which 10 required vascular surgery (4 arteriovenous fistulas, 4 pseudoaneurysms, and 2 hemorrhages with hematoma).
Procedure Variables
| Variable | All (n = 598) |
|---|---|
| Device size, median [25th-75th percentile] | 24 [22-27] |
| Need to change initial device | 60 (10.0) |
| Implant success | 573 (95.8) |
| Complications | 30 (5.0) |
| Death | 1 (0.17) |
| Stroke | 5 (0.8) |
| Vascular surgery | 10 (1.7) |
| Tamponade | 12 (2.0) |
| Migration device | 2 (0.4) |
| Otorhinolaryngologic bleeds | 3 (0.5) |
| Mitral regurgitation | 1 (0.2) |
Unless otherwise indicated, data are expressed as No. (%).
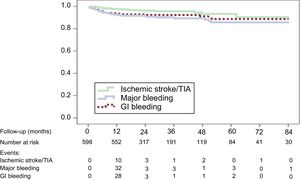
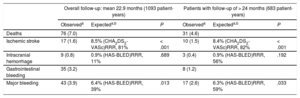
Table 4 shows the main events during follow-up for the overall group (with successful LAA closure device implantation; mean follow-up duration 22.9 months, median 16.1 months) and the subgroup with follow-up greater than 24 months.
Overall Event Outcomes in Patients With Successful Implantation, and in Those With > 24 Months of Follow-up
| Overall follow-up: mean 22.9 months (1093 patient-years) | Patients with follow-up of > 24 months (683 patient-years) | |||||
|---|---|---|---|---|---|---|
| Observeda | Expecteda,b | P | Observeda | Expecteda,b | P | |
| Deaths | 76 (7.0) | 31 (4.6) | ||||
| Ischemic stroke | 17 (1.6) | 8.5% (CHA2DS2-VASc)RRR, 81% | < .001 | 10 (1.5) | 8.4% (CHA2DS2-VASc)RRR, 82% | < .001 |
| Intracranial hemorrhage | 9 (0.8) | 0.9% (HAS-BLED)RRR, 11% | .689 | 3 (0.4) | 0.9% (HAS-BLED)RRR, 56% | .192 |
| Gastrointestinal bleeding | 35 (3.2) | 8 (1.2) | ||||
| Major bleeding | 43 (3.9) | 6.4% (HAS-BLED)RRR, 39% | .013 | 17 (2.6) | 6.3% (HAS-BLED)RRR, 59% | .033 |
RRR, relative risk reduction.
Unless otherwise indicated, data are expressed as No. (%).
The outcomes in patient-years in the overall sample and in the subgroup of patients with follow-up > 24 months were, respectively: deaths, 7.0% and 4.6% (P < .001); ischemic stroke, 1.6% and 1.5% (expected according to score: 8.5%); intracranial hemorrhage (ICH), 0.8% and 0.4% (P = .297); gastrointestinal bleeding, 3.2% and 1.2% (P < .030); and major bleeding, 3.9% and 2.6% (P < .006) (expected: 6.3%). The event-free survival curves for these clinical events are shown in Figure. The number of patients followed up at each time point is indicated along with the reduction of events after the first year.
In the multivariate analysis (Cox regression), ICH (hazard ratio [HR], 6.8; 95% confidence interval [95%CI], 2.1-22.0; P = .001), age (HR, 1.1; 95%CI, 1.0-1.1; P < .001) and stroke during follow-up (HR, 2.7; 95%CI, 1.3-5.7; P = .009), but not gastrointestinal bleeding, were associated with higher mortality.
Thrombus was found in the device in 27 patients (4.7%). These patients had a higher incidence of stroke (11.1% vs 2.6%; P = .041). The incidence of thrombus was significantly higher with the Amplatzer Cardiac Plug device (7.6%) than with Amulet (2.4%; P = .019) or Watchman (0.9%; P = .013). No differences were detected comparing Amulet and Watchman (P = 1.000).
DISCUSSIONOur series included 573 patients with a contraindication for NOAC treatment, most of whom also had a history of major bleeding, who had undergone successful LAA closure device implantation, with a mean follow-up of 22.9 months (1093 patient-years). Of these, 176 had follow-up > 24 months, with a mean follow-up of 46.6 months (683 patient-years of follow-up).
Thus, the main contribution of our study is to explore the mid- to long-term follow-up of the events (thromboembolic and bleeding), of the overall series, with a mean follow-up of almost 2 years, and a subgroup with follow-up > 24 months. Most large registries to date have only been able to compare patients with mean follow-up durations of about 1 year. After multivariate analysis, 3 variables emerged as predictors of long-term mortality: ICH, stroke, and age.
The first finding to highlight is that, just as study populations that can be randomized to receive OACs show major bleeding rates of between 3 to 4 per 100 patient-years,18 in patients in whom LAA closure was requested in real life—with high HAS-BLED scores and contraindications for OACs—major bleeding rates were 3.9% per 100 patient-years, this being especially due to patients with gastrointestinal bleeding. Of note, the HAS-BLED score is based in the bleeding risk of patients who can use OAC. Notably, a history of gastrointestinal bleeding was the main predictor of a new gastrointestinal bleed during follow-up (HR, 4.27; 95%CI, 1.87-9.73; P = .001), a finding previously reported by Witt et al.19
The effect of LAA closure is especially significant in reducing bleeding as longer follow-up periods are obtained, such that only 2.6% of patients in the population with follow-up > 24 months had major bleeding, despite the high risk in this population. Reductions above the expected for the HAS-BLED score were 59% in patients with follow-up > 24 months and 39% in the overall series, respectively. This finding is particularly relevant, as the patient-year bleeding rate in studies with OAC/NOAC have remained constant over the years.18,20 Thus, the RELY-ABLE study was an extension of the RELY study, in which only 48% of patients who took dabigatran in the original study were included; these were precisely those who had fewer previous bleeds and a lower bleeding risk. Despite these low risk determinants, the rate of major bleeding in a follow-up of > 2 years was 3.74%.20
Our results agree in this aspect with those of the Multicenter Registry, although our study has a longer follow-up time.11 In the Multicenter Registry, an analysis was performed in patients treated with aspirin alone or no treatment, and compared according to whether follow-up was greater or less than 1 year. Both groups had a HAS-BLED score of 3.2 and a mean expected risk of bleeding of 5.64. In the group with follow-up > 1 year (mean of 22.8 months [15.5-30.4]), major bleeds were 1.2% vs 4.1% in the group with follow-up < 1 year (mean of 6.3 months [4.2-8.8]) (P < .05), and the reductions above the expected for the HAS-BLED score were 90.1% vs −36.2% (P < .001), respectively. Thus, the period of 6 months to 1 year is especially critical, after which the reductions in bleeding are very significant.
This also applies to patients who were able to take OACs according to a meta-analysis of randomized studies with the Watchman device and warfarin, with a mean follow-up of 3.1 years. In this study, a similar rate of major bleeding can be seen: 3.5 vs 3.6 per 100 patient-years. However, when procedure-related bleeding during the first 7 days was excluded, the bleeding rates were 1.8 vs 3.6 events per 100 patient-years in favor of LAA closure (rate ratio [RR], 0.49; P = .001), being especially marked beyond 6 months after reducing the thrombotic treatment (1.0 vs 3.5 events per 100 patient-years; RR, 0.28; P < . 001).This was the case for all at-risk patient subgroups, regardless of the HAS-BLED score.21
The reductions in ischemic stroke remained very significant from as early as the first year and, more importantly, this effect was maintained in the more long-term results (1.6% and 1.5% between both populations vs 8.4% expected, which represents reductions of 81% and 82%, respectively, in the overall series and in populations with follow-up periods greater than 2 years). The agreement of these findings with the various registries on these types of patients reinforces the reliability of these results.8–11 Thus, in the Multicenter Registry, the rates of stroke in patients with follow-up periods of < 1 vs > 1 year were 3.77% vs 1.03%, with reductions as expected (5.62%) for the CHA2DS2-VASc score of 33% vs 81%.11
In the randomized PROTECT-AF study with long-term follow-up, a reduction in mortality was observed in those patients with LAA closure vs warfarin treatment,22 although no predictors of mortality were reported. In our study, multivariate analysis identified ICH during follow-up (HR, 6.8; 95%CI, 2.1-22.0; P = .001), age (HR, 1.1; 95%CI, 1.0-1.1; P < .001), and ischemic stroke during follow-up (HR, 2.7; 95%CI, 1.3-5.7; P = .009) as risk factors associated with higher mortality. The main predictors of ICH and stroke in the age-adjusted multivariate analysis were having experienced these events before the procedure (odds ratio [OR], 5.03; 95%CI, 0.92-27.5; P = .062 for ICH; and OR, 9.97; 95%CI, 2.28-43.47; P = .002 for recurrent stroke), which was often the main indication for LAA closure in these patients.
Intracranial hemorrhage rates were 0.87 vs 0.4 per 100 patient-years in the overall population and in patients with > 24-months of follow-up, representing a 4-fold reduction, with a 56% reduction in accordance with that expected for the score (0.9 per 100 patient-years) in the latter group.
Intracranial hemorrhage in patients with nonvalvular atrial fibrillation and NOAC treatment has also been shown to be a predictor of mortality in studies of various registries. Two recently published registries including patients with nonvalvular atrial fibrillation and NOAC treatment reported that the risk of stroke and mortality following ICH is higher in these patients.23,24 Thus, in the American Registry with 2 084 735 patients with atrial fibrillation, 50 468 (2.4%) developed ICH and 89 594 (4.3%) developed stroke during a follow-up of 3.2 years, with the annual cumulative rate of stroke being 8.1% after ICH, 3.9% after subdural hemorrhage, and 2% in those with no previous ICH.23 These results are very similar to those of the Danish registry, in which 58 815 patients with nonvalvular atrial fibrillation were studied. The group with ICH had an increased risk of stroke during follow-up, with OR 3.67, and 5.55 for mortality. The RR of claimed warfarin prescriptions post- and pre-ICH events was 0.28, which could affect these results.24
An analysis of patients with ICH in the European Registry has shown better outcomes in these patients following LAA closure than expected, based on the scores.25 Thus, in 198 patients with a mean follow-up of 1.3 years, the observed rate of stroke/transient ischemic attack was 1.4%, with a 75% reduction in the risk above that expected based on the CHA2DS2-VASc score. Cruz-González et al.26 also found percutaneous LAA closure was safe and effective in patients with an indication for long-term anticoagulation for nonvalvular atrial fibrillation and a history of ICH.
Finally, the best predictor of stroke during follow-up was having experienced a previous stroke; however, and consistent with a recently published meta-analysis, the appearance of a thrombus in the device during follow-up was also associated with a higher predisposition to new strokes.27 However, there is some controversy regarding this aspect, with results differing according to the study.28
Unfortunately, due to the lack of homogeneity of the hospitals in the follow-up with TEE, we were unable to accurately establish the time of onset of the echocardiographic findings in relation to the thromboembolic events. Nevertheless, hospitals with longer follow-up seem to conclude that it peaks between the third and sixth month.29 The relationship with antithrombotic treatment is suggestive only, as treatment guidelines vary according to the patient's risk profile, although most followed a dual antiplatelet regimen for between 3 and 6 months and aspirin therapy thereafter.
LimitationsAmong the limitations of our study are those inherent to a registry analysis. However, most of the participating hospitals and investigators included at-risk patients and very similar indications, with comparable outcomes that reflect the present state of this technique. The number of TEE follow-up studies differs according to the protocols of the different hospitals, but at least 2 studies were conducted in most of them (between 1-3 months and between 3-6 months). Finally, the postimplantation antithrombotic treatment was similar but varied, partly reflecting the absence of clear indications in this field, and also reflecting that the regimen can be altered in each patient according to their risk profile.
CONCLUSIONSLeft atrial appendage closure is being performed in very high-risk patients in relation to those included in NOAC studies, resulting in higher patient-year rates of bleeding in the first and second year. Despite this, our series, with a large number of patients under long-term and on very long-term follow-up (> 24 months) shows that the bleeding rates are lower, even than those reported in randomized studies with NOACs in patients with lower bleeding risk. The reduction in stroke rates is significant from the first year, and this beneficial effect is maintained in follow-up periods of > 24 months. The main predictors of mortality are age, previous ICH, and the occurrence of stroke during follow-up. The latter event could be improved with strategies that reduce the appearance of thrombi in the device.
CONFLICTS OF INTERESTJ.R. López-Mínguez, D. Arzamendi-Aizpurua, and V. De Gama Ribeiro are proctors of St. Jude Medical/Abbot for LAA closure with Amplatzer Cardiac Plug/Amulet; E. Infante De Oliveira, and R. Ruiz-Salmerón are proctors of Boston Scientific with Watchman device.
- –
Left atrial appendage closure is a therapeutic option for patients with nonvalvular atrial fibrillation and a contraindication for anticoagulants. Randomized studies have shown a reduction in mortality and thromboembolic/bleeding events with LAA closure. Although guidelines assign LAA closure a class IIb indication in this context, this recommendation is not shared by some investigators when it refers to patients with a contraindication for OAC, or in clinical practice by physicians who treat patients with different types of contraindications for OAC in real life. Therefore, analysis of outcomes in real-world practice is paramount
- –
In this population, LAA closure significantly reduced the incidence of strokes from the first year after implantation. For bleeding events, the reduction became significant with a longer follow-up, mainly due to the high incidence of gastrointestinal bleeding in the first year. Intracranial hemorrhage, age and a stroke during follow-up were associated with higher mortality.