Interest in the thrombogenic role of the left atrial appendage extends back to 1949, when Madden performed the first ever surgical excision of this anatomical structure.1 A growing body of literature during the past half century, along with the development of less invasive, percutaneous strategies for left atrial appendage closure (LAAC) over the past 2 decades, have fuelled widespread use of LAAC for stroke prevention in patients with nonvalvular atrial fibrillation (AF), particularly in those at high bleeding risk.2 However, while remarkable improvements have been reported in procedural outcomes over time due to increasing operator expertise and unceasing technological iterations,3 very limited data exist on very long-term outcomes following LAAC.4,5
In a recent article published in Revista Española de Cardiología, López-Mínguez et al.6 report the long-term results of the Iberian Registry II in patients with contraindications to anticoagulation who underwent LAAC. The study included 167 patients from the previous Iberian Registry I7 plus 431 additional patients enrolled thereafter, leading to a study population of 598 patients (median age: 75 years, median CHA2DS2-VASc: 4, median HAS-BLED: 3). Of these, 487 (81%) and 111 (19%) patients underwent LAAC with the Amplatzer Cardiac Plug/Amulet (Abbott Vascular, Santa Clara, CA, United States) and WATCHMAN (Boston Scientific, Natick, MA, United States) devices, respectively. The rates of successful device implantation and major periprocedural complications were 95.8% and 5.0%, respectively. After a mean follow-up of ∼ 2 years, the rates of death, ischemic stroke, and major bleeding events were 7.0, 1.6, and 3.9 patient-years, respectively. This represented a decrease of 81% and 39% with respect to the expected rate of ischemic stroke and major bleeding events according to thromboembolic and hemorrhagic risk scores, respectively. Of note, very long-term follow-up data (> 2 years, mean of ∼ 4 years) was available in 29% of patients, with similar efficacy results regarding stroke reduction, albeit with a notably greater reduction (up to ∼ 60%) in major bleeding events. The incidence of device thrombus formation was 4.7%, as evaluated by transesophageal echocardiography (TEE) within the 6 months following the procedure, and device thrombosis determined an increased risk of stroke during follow-up. Age, intracranial hemorrhage, and stroke were associated with increased mortality during follow-up.
The prospective and real-world nature of this large registry, with a mean follow-up of ∼ 4 years in more than one fourth of patients, undoubtedly represents the major strength of López-Mínguez's work. However, some limitations should be acknowledged. First, although multicenter real-world studies are generally subject to variability in follow-up according to the practice of local institutions, data on the timing and number of clinical follow-up visits are lacking, and no information is provided on missing data/patients lost to follow-up. Second, detailed information on postprocedural antithrombotic therapy is also lacking, assuming 3 to 6 months of dual antiplatelet therapy in all patients followed by aspirin for at least 6 to 12 months. However, the same authors reported that> 10% of patients had received antithrombotic treatment other than dual antiplatelet therapy in the Iberian Registry I (which represented 28% the patients included in the current study). Third, although rigorous surveillance imaging within 6 months postLAAC was stated (and certainly commendable), a 100% TEE surveillance is unlikely, and the authors failed to report the real percentage of patients who had a TEE performed within the months following the procedure. Additionally, data on residual leaks was not recorded, and no centralized evaluation of TEE images was available. Finally, the lack of both monitoring and independent event adjudication represents inherent limitations of this type of registries, which may indeed translate into an underestimation of the real incidence of clinical events over time.
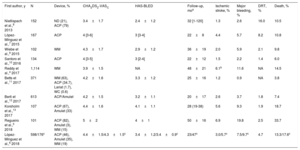
Several studies have reported long-term safety and efficacy data with the most commonly used LAAC devices (Table 1).4–13 However, studies with data beyond 4 to 5 years have been very scarce.4,5 The long-term death rate following LAAC has ranged from 3.8% to 33.7%, with wide variability according to the duration of follow-up (ranging 20 to 50 months).4–13 Indeed, most patients undergoing LAAC nowadays are elderly, with contraindications to anticoagulation, and very often exhibit a high burden of comorbidities leading to a substantial increase in mortality risk. The rate of all-cause death observed in the Iberian Registry II is slightly higher than that reported in most observational studies with similar follow-up,4,7–12 but lower than that reported by Korsholm et al.13 and Regueiro et al.5 Of note, Regueiro's work included the highest risk profile population with the longest follow-up reported to date (up to 8 years),5 which may partially explain these differences. Larger studies are needed to appropriately evaluate the risk factors associated with increased mortality following LAAC and identify those patients for whom a preventive therapy like LAAC may be futile.
Studies on Long-Term Follow-up After Left Atrial Appendage Closure
| First author, y | N | Device, % | CHA2DS2-VASC | HAS-BLED | Follow-up, moa | Ischemic stroke, % | Major bleeding, % | DRT, % | Death, % |
|---|---|---|---|---|---|---|---|---|---|
| Nietlispach et al.,8 2013 | 152 | ND (21), ACP (79) | 3.4±1.7 | 2.4±1.2 | 32 [1-120] | 1.3 | 2.6 | 16.0 | 10.5 |
| López-Mínguez et al.,7 2015 | 167 | ACP | 4 [3-6] | 3 [3-4] | 22±8 | 4.4 | 5.7 | 8.2 | 10.8 |
| Wiebe et al.,9 2015 | 102 | WM | 4.3±1.7 | 2.9±1.2 | 36±19 | 2.0 | 5.9 | 2.1 | 9.8 |
| Santoro et al.,10 2016 | 134 | ACP | 4 [3-5] | 3 [2-4] | 22±12 | 1.5 | 2.2 | 1.4 | 6.0 |
| Reddy et al.,4 2017 | 1,114 | WM | 3.9±1.5 | NA | 48±21 | 6.1b | 11.6 | NA | 14.5 |
| Betts et al.,11 2017 | 371 | WM (63), ACP (34.7), Lariat (1.7), WC (0.6) | 4.2±1.6 | 3.3±1.2 | 25±16 | 1.2 | 0.9 | NA | 3.8 |
| Berti et al.,12 2017 | 613 | ACP/Amulet | 4.2±1.5 | 3.2±1.1 | 20±17 | 2.6 | 3.7 | 1.8 | 7.4 |
| Korsholm et al.,13 2017 | 107 | ACP (67), Amulet (33) | 4.4±1.6 | 4.1±1.1 | 28 (19-38) | 5.6 | 9.3 | 1.9 | 18.7 |
| Regueiro et al.,5 2018 | 101 | ACP (82), Amulet (3), WM (15) | 5±2 | 4±1 | 50±16 | 6.9 | 19.8 | 2.5 | 33.7 |
| López-Mínguez et al.,6 2018 | 598/176c | ACP (46), Amulet (35), WM (19) | 4.4±1.5/4.3±1.5c | 3.4±1.2/3.4±0.9c | 23/47c | 3.0/5.7c | 7.5/9.7c | 4.7 | 13.3/17.6c |
ACP, Amplatzer Cardiac Plug; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes, stroke history, vascular disease, sex; DRT, device-related thrombosis; HAS-BLED, hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly); NA, not available; ND, nondedicated devices; WC, WaveCrest; WM, WATCHMAN.
One of the most notable findings of the Iberian Registry II was the substantial sustained efficacy in thromboembolic prevention (compared with the risk estimated by thromboembolic risk scores in historical cohorts), which constitutes the key rationale for LAAC. The low stroke rate observed in this registry compares favorably with that reported in most previous studies.4,5,7,12,13 Similarly, the 1.5% annual stroke rate (cumulative rate, 5.7%) observed among the subset of patients with follow-up> 2 years was similar to that reported in the combined 5-year outcomes of the PROTECT AF (Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the WATCHMAN LAAC Device in Patients With AF Versus Long-Term Warfarin Therapy) trials,4 and Regueiro's work, also with a mean follow-up of ∼ 4 years.5 The ongoing ASAP-TOO (Assessment of the WATCHMAN Device in Patients Unsuitable for Oral Anticoagulation, NCT02928497) trial will randomize about 900 patients with nonvalvular AF ineligible for anticoagulation to LAAC with the WATCHMAN device or medical treatment (single antiplatelet therapy or no treatment) in a 2:1 design, and will provide definitive evidence on the long-term efficacy of LAAC in this challenging population.
Major bleeding events occurred in 7.5% of patients in the overall population and in 9.7% of those patients with> 2-year follow-up (annual bleeding rate of 3.9% and 2.6%, respectively), with a clear tendency toward a reduction in bleeding events among patients with extended follow-up. However, this represents a much higher incidence compared with that of ischemic stroke, with most bleeding events being of gastrointestinal origin. Of note, the rate of intracranial hemorrhage 2 years after LAAC was similar to that expected based on bleeding risk scores (0.8 vs 0.9 per 100 patient-years, respectively), suggesting a potential harmful effect of dual antiplatelet therapy used within the months following the procedure. Indeed, intracranial hemorrhage was associated with an increased mortality risk at follow-up. The bleeding rate observed among patients with a mean follow-up of 4 years was similar to that reported in the 5-year outcomes of the PROTECT AF and PREVAIL trials, although the latter included a lower risk population (eligible for anticoagulation). The progressive reduction in the risk of major bleeding over time observed in the Iberian Registry II is of utmost importance, particularly considering the high-risk profile of the study population (median HAS-BLED of 3, with 70% of patients with a history of bleeding), suggesting that the increased risk of early hemorrhagic events could be partially related to the use dual antiplatelet therapy following LAAC. Further randomized studies are warranted to elucidate the optimal antithrombotic therapy for preventing ischemic stroke while not increasing bleeding events following LAAC.
Device-related thrombosis following LAAC remains a major concern, with rates varying from 1% to 17%.3 López-Mínguez et al.6 reported a device-related thrombosis rate of 4.7% as evaluated by TEE 3 to 6 months postprocedure, which was more common with the Amplatzer Cardiac Plug (7.6%) than with the Amulet (2.4%) or WATCHMAN (0.9%) devices. These findings are supported by prior observations suggesting higher rates of device-related thrombosis with the Amplatzer Cardiac Plug device due to an uncovered and high-profile proximal pin connector more prone to thrombosis.14 However, a recent registry reported contradictory results, with a much higher rate of device thrombosis among those patients receiving the Amulet device.15 Importantly, device-related thrombosis was associated with a higher risk of ischemic stroke during follow-up (11.1% vs 2.6%; P=.041), similar to the results obtained in 2 large recent studies.15,16 However, López-Mínguez et al. failed to specify the antithrombotic treatment at the time of device thrombosis, and the heterogeneity on the timing of postprocedural surveillance imaging along with a lack of an independent adjudication for device thrombosis partially attenuated the value of these findings. Hence, whereas the optimal antithrombotic regimen following LAAC is already being assessed in several ongoing randomized trials, prospective studies evaluating the association between device-related thrombosis and subsequent stroke, as well as the efficacy and duration of oral anticoagulation for device-related thrombosis resolution, are urgently needed.
The ultimate goal of LAAC in patients with nonvalvular AF ineligible for anticoagulation therapy is to prevent thromboembolic complications while maintaining a low bleeding risk over time. The results presented by López-Mínguez et al. are certainly reassuring with respect to the long-term efficacy and safety of LAAC is such patients, adding valuable information to the previous real-world evidence in this field. However, data with a much larger number of patients and longer follow-up are needed, along with head-to-head comparison between different commercialized and emerging devices, and a better understanding of the clinical relevance of device-related thrombosis and residual leaks on long-term outcomes. Additionally, evidence-based data are urgently required on antithrombotic management following LAAC. Finally, the results of ongoing trials will provide definitive evidence on the role of LAAC as a nonpharmacological therapy for preventing thromboembolic events in this high-risk population.
CONFLICTS OF INTERESTJ. Rodés-Cabau has received institutional research grants from Boston Scientific. L. Asmarats has not reported any potential conflict of interest with respect to the content of this article to disclose.
L. Asmarats was supported by a grant from the Fundación Alfonso Martín Escudero, Madrid, Spain. J. Rodés-Cabau holds the Canadian Research Chair Fondation Famille Jacques Larivière for the Development of Structural Heart Disease Interventions.