Several biomarkers have been used for evaluation and quantification of myocardial injury after effective ablation. We studied possible different thermal stability and usability of the proteins released by cardiac cells injured by different energy sources.
MethodsFirstly, we tested in vitro thermal stability of creatinine kinase (CK), myocardial bound creatinine kinase (CKMB), cardiac troponins I (cTnI) and cardiac troponins T (cTnT) in collected blood samples from 15 patients (pts) with confirmed ST-segment elevated myocardial infarction (STEMI). Secondly, the biomarkers were collected and analyzed in 82 pts treated with radiofrequency ablation (RFA) and in 79 pts treated with cryo-balloon ablation (CBA).
ResultsIn vitro experiment showed that all biomarkers were stable in low temperature of −30°C. Troponins were stable in the high temperatures analyzed. A substantial drop in CK and CKMB levels were measured at 50°C and 40°C, respectively. In vivo study showed that the increase in CKMB levels was highly significant in CBA pts only. Pathological CKMB values were observed in 24% of RFA pts and 98% of CBA pts. Pathological cTnI values were observed in all pts and the rise in cTnI levels was highly significant in both groups after ablation.
ConclusionsBoth in vitro and in vivo results show that CKMB cannot be used for quantitative determination of myocardial injury produced by radiofrequency energy. Only cardiac troponins reflect myocardial injury, regardless of energy source, and may be considered in future studies for comparison of biomarkers effects of cryo versus radiofrequency ablation.
Keywords
Radiofrequency (RF) current is a common energy source used to perform percutaneous transluminal catheter-based pulmonary vein isolation (PVI) in a vast majority of patients (pts) with atrial fibrillation (AF). In recent years, cryo-balloon technique has offered a new modality to accomplish PVI.1,2,3 As several markers have been proven to be useful for diagnosis and evaluation of size of myocardial injury after acute ischemic episode, many authors tried to implement different biomarkers to evaluate and quantify the size of effective ablation lesions.4,5,6,7,8,9,10,11,12
Tissue ablation creates immediate myocardial necrosis and the process is usually slower in ischemic events. As a result the release of myocardial injury markers starts earlier.5,6 The additional problem that appears when analyzing biomarkers released after ablation-related injury is the influence of low or high temperature on their stability. It is still unknown whether reaching the effective intra-tissue temperature of 42°C during ablation can or cannot lead to denaturing of biomarkers structure.13 Thus the results of biomarkers activity and concentration would not be reliable any more in such a setting. The only relevant paper, published in 1995, describes inactivation of creatinine kinase (CK) at the temperature of 65°C.14
Both past and recently published studies used CK, myocardial bound creatinine kinase (CKMB) and cardiac troponins I (cTnI) and T (cTnT) to evaluate the myocardial cells injury after ablation.4,5,6,7,8,9,10,11,12 Some of the results produced concerns about reliability of measurements showing inadequately small rise in CK and CKMB after effective radiofrequency ablation (RFA). Therefore we tested the hypothesis of possible different thermal stabilities of the proteins released by cardiac cells after being injured by different energy sources. Moreover, we found only single reports describing in vitro stability of biomarkers at different temperatures.15,16 Knowing the influence of different temperatures on biomarkers values would let us better understand the effectiveness or failure of ablation in certain pts.
The first step of the study was to test, in a joint in vitro experiment, the thermal stability of the studied cardiac biomarkers. The second aim was to clarify which of the biomarkers are usable for evaluation, quantification and comparisons of lesions produced by different energy sources. We applied measurements of the blood concentration of cardio-specific biomarkers, before and after ablation, as surrogate parameters for the injured cell mass.
Methods In Vitro Study of Thermo Stability and Thermo Resistances of the Cardiac BiomarkersWe collected blood samples of 10ml each from 15 pts with confirmed ST-segment elevated myocardial infarction (STEMI). Median time to the index event of STEMI was 13.7h.
After centrifugation for 15min at 3000rpm, the supernatant serum was harvested and CK activity, CKMB, and serum concentration of cTnI and cTnT were measured at 37°C within 1h. This measurement was defined as the baseline. Each pt was his own control.
The remaining serum samples were stored at −30°C for the next 2 weeks. After that period of time the probes were thawed and analyzed once more at 37°C to study the influence of freezing. Another 5 samples from each of the pts were separately incubated in a water bath at 40°C, 45°C, 50°C, 55°C and 60°C each for 5minutes. Final concentration measurement followed at 37°C.
We used standard laboratory kits: CL NAC, CKMBL, Troponin T (Roche®) and STAT Troponin I (Abott®). The reproducibility of concentrations for all investigated biomarkers, obtained by triple samples, was ±10%. The quality of the results is confirmed by the biannual quality control of the central biochemistry laboratory of our heart center.
In Vivo StudyThe study population consisted of 161 consecutive pts with symptomatic and drug refractory AF which was documented, in at least two electrocardiograms, in the three months preceding an ablation procedure. The indication for ablation was based on the current guidelines.17 Pts with primarily unknown or known elevated levels of any of the investigated markers (cTnI, CKMB) at baseline were excluded from the study.
The procedure-related risk was presented in detail and written informed consent was obtained from all pts before the ablation. The study was approved by the local ethics committee. The general characteristics of the study population are given in Table 1.
Table 1. Patients Characteristics *
| RF-group | Cryo-group | P | |||||
| Median | 25 | 75 | Median | 25 | 75 | ||
| Patients | 82 | X | X | 79 | X | X | ns |
| AFP [n] | 48 | X | X | 76 | X | X | <.001 |
| Male [n] | 61 | X | X | 49 | X | X | ns |
| Age [years] | 57 | 49 | 65 | 56 | 49 | 63 | ns |
| TTE long axis [mm] | 56 | 51 | 61 | 53 | 48 | 56 | .002 |
| TTE short axis [mm] | 40 | 37 | 42 | 38 | 35 | 41 | .01 |
| TTE LVEF [%] | 60 | 55 | 62 | 60 | 58 | 65 | .004 |
| Hypertension [n] | 53 | X | X | 42 | X | X | ns |
| CAD [n] | 10 | X | X | 7 | X | X | ns |
| BMI [kg/m2] | 27 | 25 | 29 | 26 | 23 | 28 | ns |
| Other heart diseases [n] | 9 | X | X | 5 | X | X | ns |
AFP, number of patients with paroxysmal atrial fibrillation; BMI, body mass index; CAD, coronary heart disease; LVEF: left ventricle ejection fraction; ns, not statistically significant; RF, radiofrequency; TTE, trans-thoracic echocardiography.
* Data express n or median (interquartile range).
We reached left atrium via double transeptal approach and made selective angiography of all pulmonary veins (PVs). RFA ablation was performed with 4-mm irrigated 7F Thermo-cool catheter (Biosense Webster, Diamond Bar, USA). The endpoint for ablation was total disappearance of PV potentials. We used the MESH catheter (high density mesh mapper, Bard Electrophysiology, Lowell, MA, USA) placed at ostium of each PV. Additional linear lesions (roof line, mitral isthmus line) were performed in chronic atrial fibrillation (CAF) pts. Cryo-ballon ablation (CBA) was performed with a double-walled balloon (Arctic Front, Cryocath). Application time was 240–360s per freeze. PVI was confirmed with Lasso catheter (Biosense Webster). The procedure was described recently in detail.3 Numbers of applications, cumulative RF energy and cumulative cryo time were also calculated and recorded.
Measurement of Biomarkers in Radiofrequency Ablation and Cryo-Balloon Ablation PatientsBlood samples were obtained during venous puncture before ablation and 1h, 6h and 24h after ablation. All serum samples were analyzed with standard laboratory kits described above. CKMB and cTnI cut-off values for diagnosis of myocardial infarction (25ng/mL, 0.01ng/mL, respectively) were treated as pathologically increased.
Statistical AnalysisParametric data are expressed as median values and interquartile range (25; 75). The Mann-Whitney U-test was used to analyze parametric data and the chi-square or Fisher's exact test for non-parametric data. P values <.05 were considered statistically significant.
Results In Vitro Stability of Biomarkers in Different TemperaturesWe found a substantial drop of −46% in CK activity measured at 50°C. Blood samples warming at 50°C and 60°C resulted in CK concentrations far below the baseline values. CKMB, having higher cardiac specificity, reacts even more to the heat exposure, with a substantial drop of −48% early at 40°C (Figure 1A) and a further drop at higher temperatures. Both troponins (cTnT, cTnI) showed only small changes in a range of ±10% at different temperature levels (Figure 1B and C). The median percentage dispersion of the data, expressing stability of biomarkers exposed to different temperatures, ranged between 8% and −12%.
Figure 1. (A) Thermal stability of myocardial bound creatine kinase (CKMB). (B) Cardiac troponin T (cTnT). (C) Cardiac troponin I (cTnI) in vitro. Data are depicted as box plots with median values and interquartile range.
In Vivo StudyPatients characteristics are presented in Table 1. RFA was performed in 82 pts: 48 with paroxysmal atrial fibrillation (PAF) and 34 with persistent or CAF.
Cryo-balloon-ablation group consisted of 79 pts, including only 3 pts with CAF. Significant differences in left atrial (LA) size and left ventricle ejection fraction (LVEF) between the groups result from, as could be expected, a higher number of CAF pts in the RFA group.
None of the pts complained about symptoms suggestive of ischemia or had clinical signs of coronary ischemic episode either before or at the end of the procedure. We did not find any changes of the ST-segment in comparing ECG tracings before, during and after the procedure.
For CKMB there was no significant increase after ablation compared to baseline in the RF ablation group (Figure 2). Only the median of samples obtained at the 6th hour was at the borderline of significance (p=.05). However, the increase in CKMB levels after CBA was highly significant at all control times. The factor comparing peak values was 2.6 times higher in the CBA group (p<.05). Pathological CKMB values were observed in 24% of RFA pts and 98% of CBA pts.
Figure 2. Comparison of myocardial bound creatine kinase (CKMB) kinetics in radiofrequency (RFA) and cryo-balloon (CBA) groups. Data are depicted as box plots with median values and interquartile range. MI – Our laboratory's routine CKMB value (25ng/mL) for detection of myocardial infarction. aNot statistically significant difference (P=.5) as compared to the baseline measurement. bStatistically significant difference (P=.005) as compared to the baseline measurement. cStatistically significant difference (P<.0001) as compared to the baseline measurement.
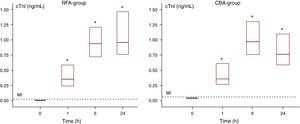
The rise in cTnI levels was highly significant in both groups after ablation (Figure 3). The median of peak values in the RF group was 1.29 (0.86; 1.86)ng/mL and it was 1.4 times higher than the respective value of CBA group 0.89 (0.62; 1.25)ng/mL. The difference failed statistical significance (p=.05). Pathological cTnI values were observed in all pts.
Figure 3. Comparison of kinetics of cardiac troponin I (cTnI) in radiofrequency (RFA) and cryo-balloon (CBA) ablation groups. Data are depicted as box plots with median values and interquartile range. *Statistically significant difference (P<.0001) as compared to the baseline measurement.
Direct current cardioversion (DCC) was performed in 19 RF and 8 CBA pts. Analyzing CK values, which hypothetically might be influenced by DCC, we found no statistically significant difference between the subjects who needed DCC to restore sinus rhythms and the rest of the studied population.
DiscussionThe main findings of our study are that pathological cTnI values were observed in all pts regardless of energy source used and pathological CKMB values were observed in 24% and 98% of RFA and CBA pts, respectively.
Cardiac Biomarkers Denaturation by TemperatureOur in vitro measurement aimed to evaluate the thermal resistances and stability of the cardiac biomarkers. We observed different temperature sensitivities of the studied biomarkers. Both cTnI and cTnT were stable in all tested temperatures.
The detected levels of CKMB and CK activity suddenly dropped to nearly 50% of base value at 40°C and 50°C, respectively. Our clinical observations are parallel to our heating experiment in vitro. The process of denaturation of the released CKMB starts at the same temperatures as the effective RFA cells injury.13 Loss of measurable concentration for CK starts at higher temperatures. Drop in sensitivity also starts within the desirable intra tissue temperature window, between 45°C and 60°C, during RFA.
The lowest temperature in our in vitro test was −30°C, which was still higher than the temperatures measured during effective CBA (usually lower than −40°C). However, this temperature is low enough to assume cell death, with rupture of the cell membranes, due to ice crystal formation that starts much earlier, at −10°C.18 We found no severe impact of low temperature on significant change in detectable levels of four studied biomarkers. Our observations are similar to described by two other authors. Buttery et al.15 confirmed stability of CKMB at −20°C. Woltersdorf et al.16 described no significant change in serum cardiac troponins concentration and CK activity. Surprisingly, CKMB concentration significantly increased in frozen samples, but the storage temperature (−70°C) was more than twice as low as in our in vitro study.
Measurement of Biomarkers in Radiofrequency Ablation PatientsOur observations are similar to those reported by others.4,5,6,7,8 Hirose found only a significant correlation for cTnT in relation to the cumulative amount of RF energy applied, but the study was limited by four different target arrhythmias with highly different numbers of applications needed for ablation in these 34 pts.4 Others6 also reported superior diagnostic accuracy of cTnI over CKMB in a non-homogenous group of pts after RFA.5,6,7 In a large cohort of 118 pts with 6 different types of arrhythmias, the only good correlation was found in regard to cTnI concentration changes.8 The mean baseline value of CKMB activity was extremely low (0.6U/l), compared to our and other reports.5,6,11 Moreover, a partial thermal deactivation of CK activity and denaturing of CKMB were assumed to be reasons for the missed correlation to RF energy delivery. His8 assumption of thermal dependency due to structural changes of CK was based, as was the case of other authors,4,5,6,7,9,10,11,12 on the Haines et al. report.14 This is the only paper in the literature describing thermal inactivation of CK in biopsies of canine hearts after RF energy ablations or after direct heating of the tissue probes. As a critical temperature he measured 65°C, which seems to be too high in comparison to our in vitro results. Intra-tissue temperature was also not measured as well as in our clinical setting.
There are also studies which showed significant increase of CKMB concentration after RFA.9,10,11 Although they seem to contradict our results, this phenomenon may be explained by low effective intra-tissue temperatures (40°C–45°C) achieved during ablation at different sides of the heart, especially in the AV-node region. The existence of a negative intra-tissue gradient beginning at the subendocardium is known. There is some likelihood that, with some of these RF applications, the critical temperature for inactivation of CK was not reached in the mass of affected myocardium.
Measurement of Biomarkers in Cryo-Ballon Ablation PatientsA single report describing myocardial injury biomarkers after cryo-ablation showed significantly higher peak values both for CKMB and cTnT in the 6th hour after ablation.12 The results are in accordance with our observation. Although we measured cTnI and not cTnT, we also observed the highest cTnI values at 6th hour blood collection from CBA pts. The authors argued that higher levels of CKMB in the cryo-group reflects different lesions formation and that bigger 10-French sheaths used in the cryo-group lead to higher values.12 Having in mind our in vitro results, we dare to say that it was rather a result of thermal instability of the biomarkers in the RFA environment.
LimitationsThe higher number of CAF pts in the RFA group, compared to the CBA group, can possibly bias the results in favor of the RF group when comparing cTnI in both groups. The detailed kinetics of biomarkers cannot be discussed, as no blood collection was performed between the 6th and 24th hour. We cannot exclude that reaching temperatures of −70°C and lower can lead to increase values of measured CKMB concentration. Nevertheless, the lowest median temperature observed during CBA in the study population was nearly −40°C.
ConclusionsBoth in vitro and in vivo results show that CKMB cannot be used for quantitative determination of myocardial injury produced by RF energy. Only cardiac troponins reflect myocardial injury regardless of energy source and may be considered in future studies for comparison of biomarkers effects of cryo versus RF ablation.
Conflict of interestMaciej Wojcik was supported by European Heart Rhythm Association (2-years Clinical Electrophysiology Fellowship). Sebastien Janin was supported by Fédération Française de Cardiologie (French Federation of Cardiology).
Received 7 February 2010
Accepted 25 August 2010
Corresponding author: Department of Cardiology, Medical University of Lublin, SPSK Nr 4, ul.Jaczewskiego 8, Lublin, Poland. m.wojcik@am.lublin.pl