To validate the axillary approach as a safe and efficient option for the transcatheter aortic valve implantation in patients who have contraindication for femoral approach at three Spanish hospitals.
MethodsWe included patients with severe symptomatic aortic stenosis at very high or prohibitive surgical risk, selected by a multidisciplinary team, for transcatheter aortic valve implantation, and had contraindication to the femoral approach.
ResultsWe included 19 of 186 (10.5%) patients, who were implanted a percutaneous aortic valve, between November 2008 and March 2010. The mean age was 78.3 (standard deviation [SD]±8.65) years and 73.7% were males. The mean logistic EuroSCORE was 28.7% (SD±16.3%). The procedural success rate was 100%. After the procedure the maximum transvalve gradient decreased from 81.7mmHg (SD±21.5) to 15.8mmHg (SD±5.5), and no patient presented residual aortic regurgitation >2. The all-cause mortality, with a mean follow-up time of 9.2 (SD±3.2) months was 10.5%, and the in-hospital and 30-day mortality rates were 0%. The global incidence of major complications due to the procedure was 15.7%. Definitive pacemaker implantation was carried out for atrioventricular block in 8 patients (44.4%).
ConclusionsThe axillary approach for transcatheter aortic valve implantation using the CoreValve® and contraindication to the femoral approach is safe and efficient for selected patients, with excellent results in terms of success implantation and in hospital and 30-day mortality.
Keywords
Degenerative severe aortic stenosis (SAS) is the most prevalent valvulopathy in the western world, with surgical valve replacement being the treatment of choice due to its widespread use and positive results.1 However, as many as a third of patients with indications for aortic valve replacement have an elevated surgical risk or some contraindication to the procedure, which prevents them from benefiting from this type of treatment.2 In recent years, the use of the transcatheter aortic valve implantation (TAVI) has increased dramatically spurred by the positive results obtained and a simplified technique, making this a valid alternative for the large subgroup of patients with elevated surgical risk.3,4 Two different models of the percutaneous valve are currently available for the percutaneous treatment of SAS: the CoreValve ReValving System (CoreValve Inc.) and the Edwards SAPIEN RetroFlex System (Edwards Lifesciences). The implant is currently placed retrograde from the femoral artery as opposed to antegrade from the femoral vein with a transseptal puncture. The latter being the method used for the first devices implanted at the start of the decade.5 Undoubtedly, the smaller calibre of the introducers required to advance the device has contributed to making the technique less complicated. Currently, the calibre for the CoreValve system is 18 French (Fr) (with 16Fr introducers under development and close to commercialization for the small valve with a valve annulus of up to 23mm) and the SAPIEN system uses 18 (recently commercialized), 22, and 24Fr calibres, depending on the valve size. However, some circumstances such as narrowing and high tortuosity, and obstructive arterial disease that occasionally occur in the femoral and iliac arteries can make this type of access impossible for TAVI. Using a transapical implant, in the case of the SAPIEN valve using the Ascendra delivery system, can solve this problem but this option is not available for the CoreValve system. The objective of this study is to present three Spanish health centres’ experience in using the percutaneous implant of the CoreValve® aortic valve prosthesis through the axillary artery in patients with contraindications for femoral access. In order to do this, we analyzed the hospital results and the results of the medium-term follow-up of patients.
Methods PopulationOur study included 19 patients recruited from three hospitals between November 2008 and March 2010. All study patients had symptomatic SAS and indications for surgery according to the clinical practice guidelines created by the European Society of Cardiology.6 Following these guidelines, patients were selected by a multidisciplinary team made up of clinical and interventional cardiologists and cardiac surgeons. We considered patients with an elevated surgical risk (logistic EuroSCORE>20%), or with technical contraindications for surgery such as porcelain aorta as possible recipients of a percutaneous valve replacement. A transthoracic and/or transesophageal echocardiogram was performed beforehand on each patient, as well as a haemodynamic study including a coronariography, ventriculography, angiography of the ascending aorta and ileofemoral sector, and left ventricle and lung pressure tests. In patients with contraindications for a femoral access, we also performed an angiography of the left axillary and subclavian arteries and located the origin of the left internal mammary artery (LIMA). Given the characteristics of the device, the patients had to meet the following anatomical characteristics: the aortic annulus must have a diameter of ≥20 and ≤27mm as measured by ultrasound, the ascending aorta 5cm above the aortic valve plane must have a diameter of ≤40mm (for the 26mm prosthesis) or ≤43mm (for the 29mm prosthesis).
We included patients that complied with these requirements that had contraindications for a femoral access, which we defined as a femoral artery <6mm (whether naturally or due to some atherosclerotic disease), or high tortuosity in the aortic–iliac segment that would impede the advance of a delivery catheter.
The exclusion criteria in our protocol were: contraindications for the administration of any of the drugs required during the procedure, haemodynamic instability, a coronary angioplasty performed within 15 days prior to the procedure, the presence of thrombus in the left cavities, recent stroke, sepsis or endocarditis, coagulopathy or bleeding diathesis, and severe mitral regurgitation with the reversal of pulmonary venous flow.
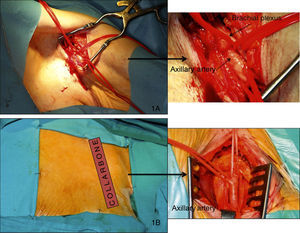
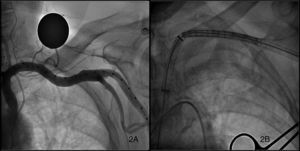
ProcedureAll procedures were performed in the haemodynamics laboratory under general anaesthesia (in this case, with early in situ extubation at the end of the procedure) or sedation with local anaesthesia, according to the protocol at each hospital. In the majority of cases, the left axillary artery (LAA) was used, and the right axillary artery (RAA) access was used in only one case. Each patient was pre-treated with 100mg/day of acetylsalicylic acid and 75mg of clopidogrel. Prophylactic antibiotics were administered according to the normal protocol in each hospital for conventional cardiac surgery, and a temporary pacemaker was placed in the right ventricle through the jugular vein before commencing the procedure. The femoral approach was used when possible, or the contralateral radial approach, for the insertion of the pigtail catheter into the non-coronary sinus of Valsalva to mark the valve annulus, and through which the contrast can be injected and arterial pressure monitored. Two different types of approaches were used to access the axillary artery, one through the axillary fossa (Figure 1A) and the other infraclavicular (Figure 1B). When the arm is extended alongside the body, the axillary artery extends obliquely downwards, laterally, and posteriorly, describing a curve with inferomedial concavity along the axillary fossa, but is straight when the arm is extended horizontally. Patients were, therefore, placed in this position when using the approach from the armpit, and a transverse incision was made at the axillary fossa. When using the infraclavicular approach, an incision was made at the axillary fossa following the long subclavian axis with the patient's arms alongside the body. Once the artery was exposed by the vascular surgeon and duly isolated with silicone surgical tape for clamping, a 9Fr valved introducer was inserted using the Seldinger technique. A 0.035in. straight guide was inserted through the introducer into the left ventricle with the help of an AL 1 catheter, which was then replaced by a 260cm super-stiff high-support guide (Amplatz Cook, Inc., Bloomington, Indiana). Finally, the 9Fr introducer was retracted and a 30cm-long, 18Fr introducer (William Cook Europe, Bjaeverskov, Denmark) was inserted through the high-support guide; when it reached the axillary artery, a small transversal arteriotomy was performed along the main arterial axis in order to avoid tearing, and the introducer was further inserted under fluoroscopic control until settling in the ascending aorta. Fluoroscopic control of the guide inserted into the left ventricle is required during this procedure in order to avoid producing myocardial damage. Once the 18Fr introducer was implanted, and after ensuring that no vascular complications had occurred, Na2+ heparin (80–100UI/kg) was administered, followed by the implantation of the valve just as in the femoral access that has been amply described in the bibliography4,7 (Figure 2A and B). In summary, the right ventricle is stimulated with a frequency above 180bpm, and after making sure that the aortic pressure curve drops and flattens, the aortic valve is dilated with a 22mm or 25mm dedicated balloon (Numed Canada Inc., Cornwall, Ontario, Canada), depending on whether the valve used is small or large. Finally, the prosthesis is released, checking that it is correctly positioned and the level of residual aortic regurgitation using fluoroscopy and angiography. Lastly, once the procedure was finished, the vascular surgeon proceeded to remove the introducer and suture the axillary artery and the zone around the site of access by layers until reaching the skin, using angiographic control to test for leaks or complications. After the procedure, the patients were moved to the coronary care unit with a temporary pacemaker where they were monitored for at least 48h in order to detect any possible heart rhythm disorders that would require a permanent pacemaker implant. The surgical wound was also cleaned daily.
Figure 1. Surgical access of the axillary artery from the axillary fossa (A) and from the infraclavicular region (B).
Figure 2. Angiography of the axillary artery (A) and the progress of the prosthesis through the introducer into the axillary artery (B).
ComplianceEach patient received a clinical, echocardiographic, and electrocardiographic follow-up one week after being discharged from the hospital, at 30 days post-discharge, and every 6 months thereafter.
Statistical AnalysisWe used the SPSS 15.0 statistical software for the analysis of our study. We performed a descriptive analysis where continuous variables were expressed as a mean and standard deviation (SD), and the categorical variables were presented as absolute values and percentages.
Results Patient CharacteristicsOf the 186 patients that received TAVI at the three hospitals, an axillary approach was used in 19 (10.2%). The mean age of the patients was 78.3 (8.65) years, and the majority were males (73.7%). The clinical characteristics and echocardiogram variables for all patients that received percutaneous valve implants, performed using both the femoral and axillary approaches, are shown in Table 1. Among these, we should point out that these were patients with an elevated surgical risk, this being significantly greater in patients with axillary access implants than those that received procedures using a femoral approach (logistic EuroSCORE: 28.7% as opposed to 18.4%, P=.003). The high prevalence of HTA (89.5%) and chronic renal failure (31.6%) also stand out, as well as a NYHA functional class greater than or equal to class III in 80% of cases.
Table 1. Basic Clinical and Echocardiographic Characteristics of the Population *
| Axillary access | Femoral access | P | |
| Patients | 19 (10.2%) | 167 (89.8%) | |
| Age (years) | 78.3 (8.65) | 81 (5.5) | .194 |
| Males | 14 (73.7) | 55 (32.9) | .001 |
| Logistic EuroSCORE (%) | 28.7 (16.3) | 18.4 (18.4) | .003 |
| BMI (kg/m2) | 28.5 (3.9) | 27.9 (5) | .607 |
| Arterial hypertension | 17 (89.5) | 122 (73.1) | .165 |
| Diabetes mellitus | 8 (42.1) | 42 (25.1) | .169 |
| Dyslipidemia | 15 (78.3) | 74 (44.3) | .006 |
| CRF | 6 (17.1) | 31 (19) | .210 |
| COPD | 10 (52.6) | 49 (29.3) | .082 |
| Revascularized ischemic cardiopathy | |||
| Percutaneous | 3 (15.7) | 23 (13.8) | |
| Surgical | 4 (21) | 11 (6.6) | |
| Both | 1 (5.3) | 1 (0.6) | |
| NYHA class>II | 15 (78.9) | 110 (82.1) | .753 |
| PCI before procedure | 3 (15.8) | 22 (13.2) | .458 |
| Aortic valve area (cm2) | 0.64 (0.19) | 0.63 (0.16) | .290 |
| Maximum gradient (mmHg) | 81.7 (21.5) | 83.6 (21.5) | .720 |
| Mean gradient (mmHg) | 47.3 (11.3) | 52.7 (15) | .133 |
| Aortic annulus (mm) | 22.7 (1.8) | 22.3 (1.8) | .427 |
| FEVI>50% | 13 (68.3) | 113 (84.3) | .108 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; EuroSCORE, European System for Cardiac Operative Risk Evaluation; FEVI, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
* Data express n (%) or mean (standard deviation).
Table 2 shows the variables related to the procedure. Two types of anaesthetic protocols were used: general anaesthesia, which was more frequent (63.1%), and deep sedation combined with infiltration of a local anaesthetic. Arterial access was primarily through the LAA (94.7%), with the RAA approach used in just one patient that was revascularized with a LIMA.
Table 2. Technical Characteristics of the Prosthetic Implant Using an Axillary Approach
| n (%) | |
| Anaesthesia | |
| General | 12 (63.1) |
| Local+sedation | 7 (36.9) |
| Arterial access | |
| Left axillary artery | 18 (94.7) |
| Right axillary artery | 1 (5.3) |
| Technique | |
| Infraclavicular | 11 (57.8) |
| Axillary | 9 (42.2) |
The results from the surgeries are shown in Table 3. The prosthesis was successfully implanted in all patients with a major reduction in maximum and mean transvalvular gradients, with no significant aortic regurgitation registered in any of the cases. These results were comparable to those obtained from patients in which the femoral approach was used for the prosthetic implantation. As for the complications that we encountered, a dissection was produced over the origin of the LIMA during the procedure for the patient that was revascularized with a LIMA to the left anterior descending artery, which was resolved using a stent. In another patient with chronic atrial fibrillation and previous episodes of stroke, a new episode of multiple embolic stroke happened 9 days after the implantation with damage to the left cerebellum and the territory dependent on the left middle cerebral artery, leaving major motor sequelae. At this point, the patient was receiving double the antiaggregation and double the low-molecular weight heparin doses than those used for antithrombotic management, which is what we believe caused this episode given the time sequence. Lastly, one of the patients had an early infectious endocarditis by Staphylococcus lugdunensis that was controlled using antibiotic treatment. A permanent pacemaker was required in almost half of the patients.
Table 3. Implant Results and Complications *
| Axillary access | Femoral access | P | |
| Patients | 19 (10.2) | 167 (89.8) | |
| Successful implant | 19 (100) | 165 (98.8) | |
| Maximum gradient (mmHg) | 15.8 (5.5) | 18.7 (6.5) | .222 |
| Mean gradient (mmHg) | 8.8 (3) | 10.2 (4.2) | .350 |
| Aortic regurgitation>level II | 0 | 0 | |
| Complications | |||
| Vascular | 1 (5.3) | 7 (4.2) | |
| Stroke | 1 (5.3) | 0 | .446 |
| Early infectious endocarditis | 1 (5.3) | 0 | |
| Need for a permanent pacemaker | 8 (44.4) | 56 (35) | |
* Data express n (%) or mean (standard deviation).
With a mean follow-up time of over nine months, mortality was 10.5% in patients with an axillary approach as compared to 14.4% in patients in which the femoral approach was used. Surprisingly, none of the patients that received surgery using the axillary approach died during the procedure, hospitalization period, or 30 days post-implant (Table 4). In all, two patients died, one of whom suddenly died little after one month following the implant. The cause of death was unclear, although it could have been due to a rhythm disorder that had previously caused left bundle branch blockade with a QRS complex width of 180ms. The second patient that died was the one that had suffered a stroke, and died from a respiratory infection six months after the implantation.
Table 4. Follow-up and Mortality *
| Axillary access | Femoral access | P | |
| Patients | 19 (10.2) | 167 (89.8) | |
| Mean follow-up (months) | 9.2 (3.2) | 9.1 (3.4) | .972 |
| Accumulated mortality | |||
| Total | 2 (10.5) | 24 (14.4) | |
| Periprocedural | 0 | 6 (3.5) | |
| Hospital | 0 | 14 (8.4) | |
| 30 days | 0 | 14 (8.4) | |
* Data express n (%) or mean (standard deviation).
We describe the approach through the axillary artery as a feasible treatment option for patients with SAS that are candidates for a percutaneous aortic valve implant and present contraindications for a femoral approach. In our study, the largest yet published on the topic, this technique showed a high success rate that was comparable or greater to that of patients from our study that received a femoral approach and those reported by other studies published on the implantation of this type of prosthesis using the femoral approach.4,8 Furthermore, it allows for an optimization of all endovascular treatment options for SAS using the CoreValve ReValving System®, since, as opposed to the other commercialized system, it does not have a delivery system for the transapical approach. In comparison with the transapical implant, the axillary approach produces less bleeding and is safer, above all in patients with severe systolic dysfunction where the transapical approach is, at least relatively, contraindicated.
In addition to the axillary or subclavian approach, several different types of TAVI accesses for the CoreValve® prosthesis in patients with contraindications for the femoral approach have been described. Among these, the transaortic approach through the ascending aorta9,10 stands out, which is performed through a ministernotomy and puncture directly over the ascending aorta. A total of three patients have received this type of procedure, with good post-implant results and no major complications. More recently, an implant was successfully placed through the left carotid artery.11 The experience related through these types of approaches is very limited, and in the authors’ opinion, they should be reserved for very select cases in which another type of approach with less bleeding is not possible. A risk/benefit analysis should always be performed, with preference for the axillary approach when a femoral access is made impossible. This will be possible in the majority of cases since even in patients with severely progressed peripheral arterial disease in the aortic–iliac segment, the subclavian–axillary system is normally wide enough to safely implant a valve. Indeed, none of the patients evaluated were rejected for having atherosclerotic disease and/or insufficient calibre. Generally, this subgroup of patients with contraindications for a femoral approach have a greater comorbidity and surgical risk; thus, the mean EuroSCORE in our sample was 28.7%, greater than in patients using the femoral approach (18.4%) and results from other studies including patients with femoral approaches, which vary between 16%4 and 25.2%,12 and in line with other studies published on patients receiving an axillary approach, such as those by De Robertis et al.13 and Modine et al.14, who reported a mean EuroSCORE of 29.5% and 34%, respectively. These results have been produced even in spite of the latest data published that point towards an underestimation of surgical risk for aortic valve replacements as defined by the EuroSCORE.15 In comparison, our intra-procedural, hospital, and 30 days post-operation mortality was 0%, with a total mortality after nine months follow-up of only 10.5%, representing more than a 50% drop in mortality from the initially predicted rates for conventional surgery. These data are also comparable or even better than those recently published for femoral implantation of this type of prosthesis,4,8,12 and those obtained in our own study in patients using the femoral approach.
Both the axillary arterial access in any of the described approaches and the later suturing by the surgeon are relatively easy, and have an advantage over the femoral approach, in which vascular complications are potentially severe. This is because once the artery is exposed, eventual problems are quickly resolved with less serious consequences, since in the extreme case of having to ligate the artery, blood flow would be maintained between the thorax and arm through the arterial anastomosis that exists between the various branches of the subclavian and axillary arteries, known as the periscapular artery circle.16 The postoperative period for an axillary approach is very well tolerated, with the advantage of being able to move around shortly afterwards whenever the patient's circumstances permit it. Another advantage of this technique is the navigability of the device; as opposed to the femoral access, the introducer is placed in the ascending aorta, and so the device reaches almost to the valve still protected by the introducer, whereas in the femoral approach, the delivery system is freely set retrograde along the aorta from its descending thoracic portion to the aortic valve plane. This sometimes causes embolism of atherosclerotic plaques, above all when passing through the crook where friction is at a maximum. For this same reason, a greater level of control is maintained over the valve than in the femoral approach, transmitting the tension/relaxation movements exerted on the delivery catheter in almost a 1:1 ratio, which provides an extra level of control over the positioning of the prosthesis. This aspect has been described by other authors13 and has also been observed in the case of the transapical access with the SAPIEN prosthesis.17 However, no evidence exists that this translates into greater success rates or better short and medium-term results.
One important aspect related to this type of approach is observed in patients revascularized with left internal mammary arteries (LIMAs). In our study, two such patients were included, one of which was treated using the RAA method, and in the other patient, who received an LAA procedure, a proximal dissection occurred in the LIMA, which was resolved using a stent without prognostic implications for the patient. The correct procedure to use in these patients is not completely clear: it constitutes an absolute contraindication for some authors,13 whereas others have published successful experiments.18 In our opinion, it need not be a contraindication, although precautions should be taken, such as a careful localization of the LIMA, and not inserting the 18Fr introducer any farther than this artery in order to avoid producing ischemia in the territory that depends upon it, and to minimise the risk of vascular complications. Furthermore, perhaps an approach through the armpit would be preferable, since this access point, being more distal than the axillary artery, allows the surgeon to advance the introducer a few centimetres farther along the artery before positioning it before the origin of the LIMA, thus providing some additional support to the system.
The incidence of stroke during the TAVI procedure is approximately 10%.8 The most frequent cause is the freeing of calcium plaque particles during the valvulopathy of the native valve or from the wall of the aorta during the advance of the device, above all in the aortic crook where major friction is produced. Avoiding this friction in the crook, and the fact that the valve advances almost until reaching the aortic valve plane while still protected by the introducer would constitute another of the advantages of the axillary approach in the reduction in number of embolisms produced. Great precautions must be taken in the manipulation of the devices and medication with antiplatelet and anticoagulant agents in high-risk patients in order to diminish the rate of stroke.
Early infectious endocarditis (IE) is an infrequent but potentially fatal complication, since the antibiotic treatment tends to be inefficient given the large anatomical substrate available for microbial colonization. The surgical treatment for IE in these high-risk patients with increased instability is often unfeasible. Therefore, these patients, in which the artery is surgically exposed using a cutaneous incision, special attention should be paid to the antiseptic measures taken and antibiotic treatment administered, since there is a greater risk of infection during the surgical procedure.
A permanent pacemaker was required in 44.4% of patients due to a high-grade atrioventricular block after the procedure, which was an incidence greater than those presented by other authors, who situate it slightly above 30%.4,19 We found no explanation for this difference based on the mode of access. Thus, we estimate that it is due to chance or conduction disturbances, since they were higher risk cases with greater comorbidities. Also, if we compare the number of patients that required a pacemaker in which the femoral approach was used in our study, this was also greater, although this result was not statistically significant (44% vs 35%, P=.446).
In the future, technological advances will make the calibre of the devices even smaller, and the proportion of patients that are excluded from using the femoral approach will be even less. However, there will always be some percentage of patients that will be able to benefit from alternative types of access, such as the axillary approach, which will improve in safety and efficiency as greater experience is gained.
ConclusionsIn our study, the axillary access for the CoreValve® percutaneous biological aortic prosthesis in patients with contraindications for a femoral approach has been shown to be a safe and efficient alternative, with comparable results to the femoral approach both in our study and in others published on the subject, in spite of this technique being used to treat patients with higher surgical risk. The use of this alternative approach increases the number of patients that can potentially benefit from this technique, which has greatly increased in recent years, prompted by these positive results.
Conflicts of interestThe authors state that they have no conflicts of interest.
Received 30 April 2010
Accepted 14 August 2010
Corresponding author: Urb. Rua Das Flores 37A. 15896, Roxos-Santiago de Compostela, A Coruña, Spain. birihh@yahoo.es