High baseline levels of interleukin-6 and C-reactive protein confer an increased risk of mortality in non-ST-segment elevation acute coronary syndrome. The aim of the study was to determine whether serial measurements of interleukin-6 and high-sensitivity C-reactive protein provide additional information to baseline measurements for risk stratification of non-ST-segment elevation acute coronary syndrome.
MethodsTwo hundred and sixteen consecutive patients with non-ST-segment elevation acute coronary syndrome were prospectively included. Blood samples were obtained within 24h of hospital admission and at 30 days of follow-up. The endpoint was a composite of all-cause death, nonfatal myocardial infarction, or acute decompensated heart failure.
ResultsBoth interleukin-6 and high-sensitivity C-reactive protein levels decreased from day 1 to day 30, regardless of adverse events (both P<.001). Interleukin-6 levels at 2 time points (interleukin-6 day 1, per pg/mL; hazard ratio=1.006, 95% confidence interval, 1.002-1.010; P=.002 and interleukin-6 day 30, per pg/mL, hazard ratio=1.047, 95% confidence interval, 1.021-1.075, P<.001) were independent predictors of adverse events, whereas high-sensitivity C-reactive protein day 1 and high-sensitivity C-reactive protein day 30 levels were not. Patients with interleukin-6 day 1≤8.24 pg/mL and interleukin-6 day 30≤4.45 pg/mL had the lowest event rates (4.7%), whereas those with both above the median values had the highest event rates (35%). After addition of interleukin-6 day 30 to the multivariate model, C-index increased from 0.71 (95% confidence interval, 0.63-0.78) to 0.80 (95% confidence interval, 0.72-0.86), P=.042, and net reclassification improvement was 0.39 (95% confidence interval, 0.14-0.64; P=.002).
ConclusionsIn this population, both interleukin-6 and high-sensitivity C-reactive protein concentrations decreased after the acute phase. Serial samples of interleukin-6 concentrations improved the prognostic risk stratification of these patients.
Keywords
.
INTRODUCTIONPrevious studies have shown high levels of both interleukin-6 (IL-6) and C-reactive protein confer an increased risk of long-term mortality in patients with non-ST-segment elevation acute coronary syndrome (NSTEACS).1–6 Moreover, early serial plasma level monitoring of these cytokines in acute coronary syndromes has also been found to be predictive of adverse outcomes,7–13 even in those patients with normal serum troponin T.14 However, most studies considered only early (24h to 72h) presenting values of these biomarkers, but the prognostic role of delayed serial measurements for long-term risk stratification in patients with NSTEACS remains uncertain.
Our objective was to evaluate the prognostic value of serial sampling of IL-6 in comparison to high-sensitivity C-reactive protein (hs-CRP), and to determine whether serial measurements provide additional information to isolated measurements in the long-term risk stratification of NSTEACS patients.
METHODSStudy Population and DesignThis study was designed to determine the prognostic utility of serial measurements of IL-6 and hs-CRP in high-risk NSTEACS patients. From 1 September 2006 to 31 March 2008, 216 consecutive patients with high-risk unstable angina or non-ST-elevation myocardial infarction were prospectively enrolled. The diagnosis of high-risk NSTEACS was established on the basis of current criteria guidelines (defined as ischemic symptoms lasting≥10min, and occurring within 72h before randomization, and either ST-segment deviation of ≥1 mm, deep symmetrical inversion of the T waves in the anterior chest leads, or elevated levels of a cardiac biomarker of necrosis). Patients with evidence of hepatic dysfunction (an alanine aminotransferase level that was more than twice the upper limit of the normal range); concomitant neoplasic, infectious, connective tissue, or inflammatory diseases; deep vein thrombosis or pulmonary embolism; and recent (<1 month) surgery or trauma were excluded, as were patients taking immunosuppressant agents.
During the entire hospitalization period, baseline clinical characteristics were prospectively recorded and all patients received standard management as recommended for NSTEACS.15,16 The clinical management decisions about each patient were decided by the cardiologist responsible who was unaware of the IL-6 or hs-CRP levels. After this, hospital discharged-patients were clinically followed up for at least 12 months—median 667 days [interquartile range, 397-944]—and adverse clinical events were obtained in all the patients. The study outcome was a composite of all cause death, nonfatal myocardial infarction, or acute decompensated heart failure during the follow-up period. Death was ascertained from available medical records and death certificates. Myocardial infarction was defined by detection of rise in cardiac biomarkers of necrosis (usually troponin T) with at least 1 value above the 99th percentile upper reference limit, together with evidence of myocardial ischemia with at least 1 of the following: electrocardiographic changes indicative of new ischemia (new ST-T changes or new left bundle branch block), new pathological Q waves in at least 2 contiguous leads, imaging evidence of new loss of viable myocardium, or new wall motion abnormality.17,18 Acute decompensated heart failure diagnosis was established on the basis of current criteria guidelines and defined as the rapid or gradual onset of signs and symptoms of heart failure, resulting in unplanned hospitalization.19 The study was approved by the local ethics committee, and informed consent was obtained from each patient at inclusion.
Biochemical AnalysisBlood samples were collected within 24h of hospital admission and at 30 days of follow-up. At 30 days post acute coronary syndrome, 146 (68%) of the 216 patients recruited attended follow-up and underwent the blood test. All blood samples were obtained prior to coronary angiogram by venipuncture at 8:00 a.m. within 24h of hospital admission and at 30 days of follow-up; aliquots of serum were stored at –80°C until analyzed. Concentrations of IL-6 and hs-CRP were determined using Cobas® 6000 analyser (Roche Diagnostics; Mannheim, Germany). Detection limits were 1.5 pg/mL for IL-6 and 0.15 mg/dL for hs-CRP. Within-run and total coefficients of variation of assays were <2% and 4.9% respectively for IL-6, and <2% and 3.6% respectively for hs-CRP.
Statistical AnalysisDifferences in population characteristics were compared using the analysis of variance or the Kruskal-Wallis test for continuous variables, and chi-square test or Fisher's exact test for categorical variables. IL-6 and hs-CRP concentrations were compared using the Wilcoxon test at the 2 time points assessed throughout the study protocol. Comparisons of both biomarkers between groups with and without events were performed by the Mann-Whitney U test. Correlations between both biomarkers and other clinical and analytical parameters were assessed by the Spearman rank correlation.
When analyzing the predictive value of both biomarker levels at day 30, events that occurred before 30 days were censored. Receiver operating characteristic curves analyses for predicting adverse clinical events were performed for both biomarkers. The best prognostic cut-off was defined as the highest product of sensitivity and specificity. To identify predictors of the composite study endpoint during the follow-up period, hazard ratios (HR) derived from the Cox regression analysis, were calculated. The independent effect of variables on the composite endpoint was calculated using a Cox multivariable regression analysis (forward stepwise), incorporating covariates with P<.05 in the univariate analysis and other covariates that are known to be associated with adverse outcomes. Log-cumulative hazard plots, time-dependent covariates, and Schoenfeld residuals were used to evaluate adherence of the Cox proportional hazard assumptions. Baseline IL-6 (IL-6day1) and baseline hs-CRP (hs-CRPday1) levels were first entered individually in the multivariable models. Subsequently, IL-6day1 were adjusted for IL-6 levels on day 30 (IL-6day30) in a separate multivariable analysis.
To test the hypothesis that serial assessment of IL-6 would improve risk stratification, patients were categorized on the basis of the number of samples with “elevated IL-6”. IL-6 samples were defined as elevated if their levels were above their median values (>8.24 pg/mL for IL-6day1 and >4.45 pg/mL for IL-6day30). A new ordinal variable was built on the basis of a combined IL-6 sample taking into account the presence of none, 1 or 2 samples with elevated IL-6 values. The IL-6 values were added separately and also as a combined biomarker score to a model containing clinical risk factors. Model performance was evaluated by C-indexes. Moreover, the improvement in predictive accuracy was evaluated by calculating the net reclassification improvement and integrated discrimination improvement, as described by Pencina et al.20 All P<.05 were accepted as statistically significant. Statistical analysis was performed by the use of SPSS version 15.0 software (SPSS Inc.; Chicago, Illinois, United States).
RESULTSThe study population consisted of 216 hospitalized patients with high-risk NSTEACS (Tables 1 and 2). IL-6day1 were positively correlated with baseline hs-CRP (hs-CRPday1) concentrations (r=0.59, P<.001). IL-6day1 weakly correlated with IL-6day30 (r=0.25; P=.002), age (r=0.24; P<.001); heart rate (r=0.16; P=.022), left ventricular ejection fraction (r=-0.16; P=.019); hemoglobin (r=−0.24; P<.001), leukocytes (r=0.22; P=.001), troponin T (r=0.30; P<.001), and GRACE (Global Registry for Acute Coronary Events) score (r=0.26; P<.001).
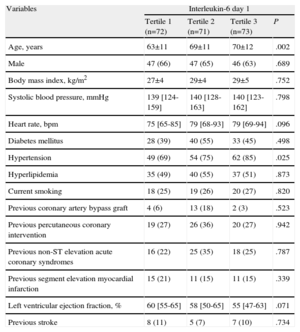
Study Population Clinical Characteristics as a Function of Baseline Interleukin-6 Levels.
| Variables | Interleukin-6 day 1 | |||
| Tertile 1 (n=72) | Tertile 2 (n=71) | Tertile 3 (n=73) | P | |
| Age, years | 63±11 | 69±11 | 70±12 | .002 |
| Male | 47 (66) | 47 (65) | 46 (63) | .689 |
| Body mass index, kg/m2 | 27±4 | 29±4 | 29±5 | .752 |
| Systolic blood pressure, mmHg | 139 [124-159] | 140 [128-163] | 140 [123-162] | .798 |
| Heart rate, bpm | 75 [65-85] | 79 [68-93] | 79 [69-94] | .096 |
| Diabetes mellitus | 28 (39) | 40 (55) | 33 (45) | .498 |
| Hypertension | 49 (69) | 54 (75) | 62 (85) | .025 |
| Hyperlipidemia | 35 (49) | 40 (55) | 37 (51) | .873 |
| Current smoking | 18 (25) | 19 (26) | 20 (27) | .820 |
| Previous coronary artery bypass graft | 4 (6) | 13 (18) | 2 (3) | .523 |
| Previous percutaneous coronary intervention | 19 (27) | 26 (36) | 20 (27) | .942 |
| Previous non-ST elevation acute coronary syndromes | 16 (22) | 25 (35) | 18 (25) | .787 |
| Previous segment elevation myocardial infarction | 15 (21) | 11 (15) | 11 (15) | .339 |
| Left ventricular ejection fraction, % | 60 [55-65] | 58 [50-65] | 55 [47-63] | .071 |
| Previous stroke | 8 (11) | 5 (7) | 7 (10) | .734 |
Data are expressed as no. (%), mean±standard deviation, or median [interquartile range].
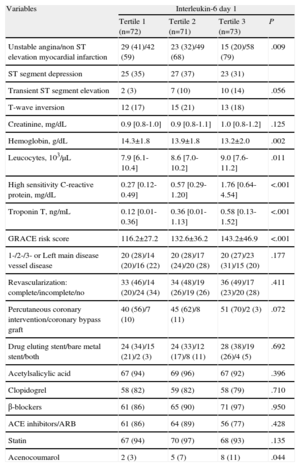
Clinical Characteristics, Laboratory, and Periprocedural Parameters as a Function of Baseline Interleukin-6 Levels.
| Variables | Interleukin-6 day 1 | |||
| Tertile 1 (n=72) | Tertile 2 (n=71) | Tertile 3 (n=73) | P | |
| Unstable angina/non ST elevation myocardial infarction | 29 (41)/42 (59) | 23 (32)/49 (68) | 15 (20)/58 (79) | .009 |
| ST segment depression | 25 (35) | 27 (37) | 23 (31) | |
| Transient ST segment elevation | 2 (3) | 7 (10) | 10 (14) | .056 |
| T-wave inversion | 12 (17) | 15 (21) | 13 (18) | |
| Creatinine, mg/dL | 0.9 [0.8-1.0] | 0.9 [0.8-1.1] | 1.0 [0.8-1.2] | .125 |
| Hemoglobin, g/dL | 14.3±1.8 | 13.9±1.8 | 13.2±2.0 | .002 |
| Leucocytes, 103/μL | 7.9 [6.1-10.4] | 8.6 [7.0-10.2] | 9.0 [7.6-11.2] | .011 |
| High sensitivity C-reactive protein, mg/dL | 0.27 [0.12-0.49] | 0.57 [0.29-1.20] | 1.76 [0.64-4.54] | <.001 |
| Troponin T, ng/mL | 0.12 [0.01-0.36] | 0.36 [0.01-1.13] | 0.58 [0.13-1.52] | <.001 |
| GRACE risk score | 116.2±27.2 | 132.6±36.2 | 143.2±46.9 | <.001 |
| 1-/2-/3- or Left main disease vessel disease | 20 (28)/14 (20)/16 (22) | 20 (28)/17 (24)/20 (28) | 20 (27)/23 (31)/15 (20) | .177 |
| Revascularization: complete/incomplete/no | 33 (46)/14 (20)/24 (34) | 34 (48)/19 (26)/19 (26) | 36 (49)/17 (23)/20 (28) | .411 |
| Percutaneous coronary intervention/coronary bypass graft | 40 (56)/7 (10) | 45 (62)/8 (11) | 51 (70)/2 (3) | .072 |
| Drug eluting stent/bare metal stent/both | 24 (34)/15 (21)/2 (3) | 24 (33)/12 (17)/8 (11) | 28 (38)/19 (26)/4 (5) | .692 |
| Acetylsalicylic acid | 67 (94) | 69 (96) | 67 (92) | .396 |
| Clopidogrel | 58 (82) | 59 (82) | 58 (79) | .710 |
| β-blockers | 61 (86) | 65 (90) | 71 (97) | .950 |
| ACE inhibitors/ARB | 61 (86) | 64 (89) | 56 (77) | .428 |
| Statin | 67 (94) | 70 (97) | 68 (93) | .135 |
| Acenocoumarol | 2 (3) | 5 (7) | 8 (11) | .044 |
ACE inhibitor; angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; GRACE, Global Registry for Acute Coronary Events.
Data are expressed as no. (%), mean±standard deviation, or median [interquartile range].
Over the study period, a total of 44 (20.3%) patients had the composite endpoint (16 deaths, 26 non-fatal myocardial infarctions and 15 acute decompensated heart failure hospitalizations). Of these, 23 (10.6%) patients had adverse events after the first 30 days (13 deaths, 21 non-fatal myocardial infarctions, and 11 acute decompensated heart failure). Median IL-6 levels were higher among patients with the composite endpoint than those without, both at presentation (15.38 [7.38-31.30] pg/mL vs 7.13 [4.29-16.35] pg/mL; P<.001) and at day 30 (8.59 [4.40-10.83] pg/mL vs 4.12 [2.67-5.80] pg/mL; P=.001). By contrast, only hs-CRPday1 levels (but not hs-CRP on day 30 [hs-CRPday30]) were higher among those with the composite endpoint (1.50 [0.36-3.99] mg/dL vs 0.53 [0.23-1.28] mg/dL; P=.009 for hs-CRPday1; and 0.39 [0.10-1.29] mg/dL vs 0.27 [0.13-0.44] mg/dL; P=.220 for hs-CRPday30).
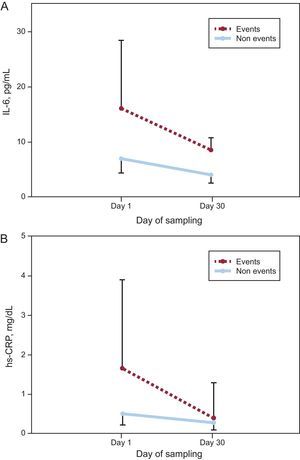
Figure 1 shows a significant decrease in concentrations of both biomarkers from presentation to day 30 regardless of adverse events, although those with events had higher concentrations of IL-6 at both times. Given the higher levels at presentation, the absolute decreases of IL-6 and hs-CRP were actually higher in patients with events (–5.87[–0.19 to –19.01] pg/mL vs –2.38[0.58 to –10.81] pg/mL; P=.019 for IL-6 and –0.72[–0.26 to –1.60] mg/dL vs –0.19[0.04 to –0.90] mg/dL; P=.009 for hs-CRP; respectively), whereas the relative changes of both biomarkers were similar in both groups (–46%[–6 to –65] vs –41%[8 to –76]; P=.627 for IL-6 and –61%[–49 to –87] vs –43%[15 to –83]; P=.190 for hs-CRP).
Change in A: interleukin-6. B: high-sensitivity C-reactive protein concentrations, from presentation to day 30 in subjects with and without adverse events. The median interleukin-6 and high-sensitivity C-reactive protein values and interquartile range (error bars) plotted against the day of sampling. hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6.
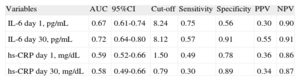
When receiver operating characteristic analyses were undertaken to evaluate the prognostic accuracy of both inflammatory biomarkers, IL-6day30 concentrations showed the best prognosis ability to discriminate between those patients with and without the composite endpoint (area under the curve [AUC]=0.72; 95% confidence interval [95%CI], 0.64-0.80), followed by IL-6day1 (AUC=0.67; 95%CI, 0.61-0.74), whilst hs-CRPday1 had overall lower prognostic accuracy (AUC=0.59; 95%CI, 0.52-0.66) (Table 3).
Performance of Measures of Interleukin-6 Levels for Prediction of Adverse Events (all Cause Death, Nonfatal Myocardial Infarction, or Acute Decompensated Heart Failure).
| Variables | AUC | 95%CI | Cut-off | Sensitivity | Specificity | PPV | NPV |
| IL-6 day 1, pg/mL | 0.67 | 0.61-0.74 | 8.24 | 0.75 | 0.56 | 0.30 | 0.90 |
| IL-6 day 30, pg/mL | 0.72 | 0.64-0.80 | 8.12 | 0.57 | 0.91 | 0.55 | 0.91 |
| hs-CRP day 1, mg/dL | 0.59 | 0.52-0.66 | 1.50 | 0.49 | 0.78 | 0.36 | 0.86 |
| hs-CRP day 30, mg/dL | 0.58 | 0.49-0.66 | 0.79 | 0.30 | 0.89 | 0.34 | 0.87 |
95%CI, 95% confidence interval; AUC, area under the curve; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; NPV, negative predictive value; PPV, positive predictive value.
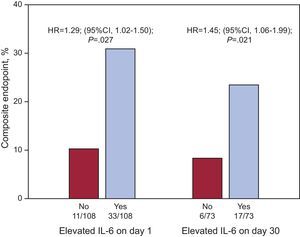
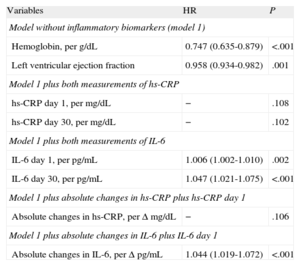
After multivariate Cox regression analyses (Table 4), IL-6 levels, as quantitative variables, were independent predictors of adverse events at the 2 time points (IL-6day1 per pg/mL, HR=1.006; 95%CI, 1.002-1.010; P=.002; and IL-6day30 per pg/mL; HR=1.047; 95%CI, 1.021-1.075; P<.001). In addition, IL-6 levels above the median values both at presentation (>8.24 pg/mL; HR=1.290; 95%CI, 1.025-1.502; P=.027) and at day 30 (>4.45 pg/mL, HR=1.453; 95%CI, 1.059-1.994; P=.021) were significantly independent predictors of events (Fig. 2), as were its absolute changes (per Δ pg/mL; HR=1.050; 95%CI, 1.024-1.077; P<.001). However, both hs-CRP concentrations and the absolute changes in hs-CRP were not independently associated with adverse outcomes (Table 4).
Cox Regression Risk Analysis for the Composite Endpoint (all Cause Death, Nonfatal Myocardial Infarction, or Acute Decompensated Heart Failure).
| Variables | HR | P |
| Model without inflammatory biomarkers (model 1) | ||
| Hemoglobin, per g/dL | 0.747 (0.635-0.879) | <.001 |
| Left ventricular ejection fraction | 0.958 (0.934-0.982) | .001 |
| Model 1 plus both measurements of hs-CRP | ||
| hs-CRP day 1, per mg/dL | − | .108 |
| hs-CRP day 30, per mg/dL | − | .102 |
| Model 1 plus both measurements of IL-6 | ||
| IL-6 day 1, per pg/mL | 1.006 (1.002-1.010) | .002 |
| IL-6 day 30, per pg/mL | 1.047 (1.021-1.075) | <.001 |
| Model 1 plus absolute changes in hs-CRP plus hs-CRP day 1 | ||
| Absolute changes in hs-CRP, per Δ mg/dL | − | .106 |
| Model 1 plus absolute changes in IL-6 plus IL-6 day 1 | ||
| Absolute changes in IL-6, per Δ pg/mL | 1.044 (1.019-1.072) | <.001 |
HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6.
The model includes those variables showing statistical significance in univariate Cox regression analyses for the composite endpoint. Also adjusted by Global Registry for Acute Coronary Events risk score, systolic blood pressure, heart rate, sex, diabetes mellitus, hypertension, creatinine, troponin T, and revascularization.
Cox regression risk dichotomized analysis for the composite endpoint (all cause death, nonfatal myocardial infarction, or acute decompensated heart failure) as a function of interleukin-6 levels above the median (>8.24 pg/mL for baseline interleukin-6 and >4.45 pg/mL for interleukin-6 levels on day 30). HR, hazard ratio; IL-6, interleukin-6.
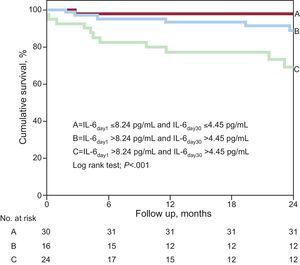
The potential enhanced value from the combined use of serial measurements of IL-6 levels for prognosis risk stratification is shown in Figure 3. This figure shows that the lowest event rate was observed in those patients with IL-6 levels below the median values at presentation and at day 30 (n=43; 4.7%; score=0), whereas the event rate observed among patients who had IL-6 levels above the cut-off values at both time points assessed throughout the study protocol was considerably higher than in any other group (n=40; 35%; score=2; P<.001). In adjusted analysis, for each measure of elevated IL-6 levels in the combined score (from 0 to 2) a significantly higher risk of events was observed (per elevated measure, HR=1.35; 95%CI, 1.090-1.670; P=.005).
Lastly, the addition of IL-6day30 concentration to a multivariable model, adjusted for other risk factors (including IL-6day1 and GRACE risk score), was associated with a significant improvement in prediction performance and accuracy. The net reclassification improvement from the addition of IL-6day30 concentrations was 0.39 (95%CI; 0.14-0.64, P=.002), whereas the relative integrated discrimination improvement was 0.49 (95%CI, 0.12-0.66; P=.001). The probability of correctly predicting events and no events when IL-6day30 was added to the clinical multivariate model was reflected particularly in the percentage of non-events correctly reclassified (48%), while the percentage of events reclassified was −9%. The C-index increased from 0.71 (95%CI, 0.63-0.78) to 0.80 (95%CI, 0.72-0.86); P=.042, with the addition of IL-6day30 to the clinical multivariate model.
DISCUSSIONIn the present study, we examined the prognostic value of serial measurements of circulating levels of IL-6 and hs-CRP in a hospitalized population with NSTEACS. Consistent with the results of earlier studies,2,21,22 we found high IL-6day1 to be associated with poor long-term outcomes. More importantly, to the best of our knowledge, we demonstrated for the first time, that serial sampling of IL-6day30 levels of NSTEACS presentation offers complementary information to its baseline levels and other clinical variables (including GRACE risk score) for long-term risk stratification. These results are novelty, considering, the use of contemporary reclassification analyses for the assessment of the prognostic value of serial measurements of IL-6 levels in this clinical scenario. Accordingly, our results support the use of repeated IL-6 testing in NSTEACS patients once the patients have been stabilized. A good strength of our study is that blood was sampled at a time when patients were considered to be clinically stabilized (30 days after presentation); yet IL-6 values were able to identify those patients who were at a lower risk for long-term adverse clinical events with high negative predictive values (>90%). In this sense, IL-6 monitoring may help us to identify patients who might not benefit from more aggressive management and monitoring.
IL-6 is a ubiquitous cytokine with a crucial role in leukocyte and endothelial cell activation that promotes increased synthesis of a wide variety of acute phase reactant proteins, including C-reactive protein, and represents the main systemic mediator of the acute response to tissue damage. Furthermore, IL-6 is a key inflammatory cytokine in atherothrombotic disorders. IL-6 is expressed at the shoulder region of atherosclerotic plaques and acts locally to enhance recruitment of monocytes and T cells, with a consequent increase in plaque instability, and acts systemically to induce a pro-inflammatory and pro-thrombotic state.23–25 In this study, we found that IL-6day1 levels are closely related to long-term prognosis of patients with NSTEACS, which reaffirms the prognostic significance of the pro-inflammatory status during the initial phase of an acute coronary syndrome.
Our results also emphasize the importance of monitoring “dynamic changes” in the inflammatory state of NSTEACS patients. Previous studies have shown that patients with unstable angina who had a decrease in IL-6 levels within 48 h to 72h of hospitalization, presented an uncomplicated in-hospital course, whereas patients with an increase in these levels had a higher rate of in-hospital14 or 30-day events.10,12 The delayed measurement of inflammatory biomarkers in assessing the long-term predictive value has 2 potential advantages. The first advantage is that we overcome the “problem” that is posed on prognosis by the easy access to revascularization, either during the hospital admission (urgently) or early after discharge (electively). This early invasive treatment in NSTEACS patients may change the natural history of coronary artery disease and thus attenuate the long-term prognostic value of an inflammatory marker measured during the acute phase. Indeed, Ozer et al. demonstrated that early coronary angioplasty modifies levels of inflammatory biomarkers, including the type of stent used.26 The second advantage is that, 4 weeks after the coronary event, the excess inflammatory response caused by the event should have decreased to the new steady state level or should be “normalized”, and inflammatory markers may then reflect the underlying chronic inflammatory process and thus, be better indicators of the severity of the disease. Consequently, a persistent elevation of inflammatory biomarkers beyond the acute phase suggests prolonged inflammation and could represent a subclinical hallmark of recurrent instability.27
In our study, IL-6 levels showed a significant decrease at 30 days regardless of the occurrence of events but measurement of IL-6day30 levels provides additional information to isolated measurements in the long-term risk stratification of these patients. It supports the hypothesis that a persistent inflammatory activation, as indicated by higher levels of IL-6, is an important predictor of long-term prognosis in patients with NSTEACS.
There are controversial results in the literature regarding the prognostic value of inflammatory markers. The SIESTA study (Systemic Inflammation Evaluation in patients with non-ST segment Elevation Acute Coronary Syndrome) evaluated the prognostic value of hs-CRP, IL-6, and other markers of inflammation in patients with low to moderate risk NSTEACS.28 In this study, inflammatory markers failed to provide independent prognostic information at 6 months and 12 months of follow-up. However, these apparently discrepant results may be attributed to differences in the population characteristics, including the higher risk profile and higher inflammatory burden of our patients (mainly reflected in higher levels of IL-6 in our population).29,30 A paper by Rallidis et al. also studied the prognostic value of cytokines monitoring after 6 weeks of presentation in patients with unstable angina, but IL-6 levels at 6 weeks were not independent predictors of long-term prognosis.13 One possible explanation could be the inclusion of the event revascularization because of new or worsening symptoms in the combined endpoint. Previous studies have failed to demonstrate an association between inflammatory markers and angina31 or inflammatory markers and late angiographic stent restenosis.32 Indeed, circulating biomarkers of inflammation may be more related to acute plaque rupture and thrombosis rather than to slowly progressive occlusive coronary aterosclerosis.33
On the other hand, our composite endpoint also included acute decompensated heart failure. Previous studies have shown that elevated IL-6 concentrations in patients with coronary artery disease preceded progression to clinically overt heart failure.34 Also IL-6 may by itself exacerbate the damage that results from minor myocardial necrosis/injury or may stimulate muscle atrophy and myocardial failure during acute coronary syndrome.35 This may be 1 of the reasons explaining why circulating IL-6 levels have been implicated on acute coronary syndrome and heart failure, and their increase is associated independently, with long-term mortality/acute decompensated heart failure in an acute coronary syndrome population.22
Our results may suggest that the prognostic value of serial IL-6 measurements may be superior to those of hs-CRP. The lack of predictive value of hs-CRP in our study contrasts with data in previous reports.3,36,37 This finding could be explained by the low statistical power of our study. However our results agree with other studies showing a limited role of hs-CRP for risk prediction. Thus, previous reports have showed that hs-CRP has added modest information to the overall prediction of risk resulting from the assessment of conventional cardiovascular risk factors.30,38 It probably suggests that hs-CRP predictive usefulness might not be universal. Further studies are needed to address this challenging issue in different populations.
LimitationsThe small sample size of patients in a single center study and the relatively small number of events, especially after the 1st month, make it difficult to draw firm conclusions. Moreover, caution must be applied when interpreting our results, as they might not be transferable to all NSTEACS patients/cases, and should be considered as hypothesis generating. Moreover, given the fact that patients with adverse clinical events within 30 days of follow-up were censored, the survivorship bias in the acute phase may limit interpretation of the results.
Economic, biological, and analytical reasons, partially explain the limitations of the use of inflammatory biomarkers. The availability of high-sensitivity and relatively low-cost methods for IL-6 measurement could facilitate its routine use. In addition, although our samples were stored at −80°C, we cannot exclude the possibility of protein degradation. Such an effect is unlikely, however, because the distribution of IL-6 in our study was quite similar to that observed in studies that used fresh blood samples. Another consideration is the lack of standardized methods for IL-6 measurement, which would include the use of the same peptide preparation and the same units, references, and cut-off values. Nevertheless, since our detection limit is 1.5 pg/mL, our automatized system allows detection within the range of the most sensitive available commercial enzyme-linked immunosorbent assay kits (0.7-5 pg/mL), and avoids variations due to sample manipulation in manual protocols. Inflammatory indexes were measured in peripheral blood, and do not allow firm conclusions on the release of these factors within the coronary circulation. Medications may affect plasma levels of the measured inflammatory indexes,39 however, patients with and without events, received similar pharmacological treatment. Another major limitation of determination of IL-6 is its low specificity, as the plasma concentrations of IL-6 are influenced by a variety of diseases. The circulating levels of IL-6 may also show a natural variability over the time-course of the disease. Indeed, IL-6 levels follow a circadian rhythm with a sharp nocturnal rise.40 To minimize this problem, blood samples were always withdrawn at the same time (at 8 a.m.).
CONCLUSIONSAmong NSTEACS patients, serial sampling of IL-6 levels at 30 days provides complementary information for the long-term risk stratification, and may help to identify patients who might not benefit from more aggressive management and monitoring.
FUNDINGThis work was partially supported by a research grant FFIS/CM09/016 from Cajamurcia and a research grant Abbott/12399 from Murcia University.
CONFLICTS OF INTERESTNone declared.