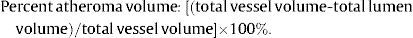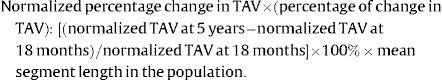The Absorb bioresorbable vascular scaffold has been shown to decrease total plaque areas in the treated segment. However, it is unknown whether plaque size is modified in scaffolded segments only or whether the modification extends to other coronary segments.
MethodsAbsorb Cohort A is a single-arm, prospective study, with safety and imaging endpoints, in which 30 patients underwent percutaneous coronary intervention with the first generation Absorb bioresorbable vascular scaffold. Noninvasive multislice computed tomography imaging was performed in 18 patients at 18 months and 5 years of follow-up. The present study was an intrapatient comparison of matched segments (normalized by the segment length) of the scaffolded region with nonintervened segments for lumen volume, vessel volume, plaque volume, plaque burden, and percent change in plaque atheroma volume.
ResultsAll 18 scaffolded segments could be analyzed. In the nonintervened segments, 1 of 72 segments had a motion artifact and was excluded. Serial comparison showed that the scaffolded segments showed no significant change in the mean plaque burden, total atheroma volume, total lumen volume, or vessel volume between 18 months and 5 years. Conversely, the untreated segments showed a significant increase in plaque burden (2.7 ± 6.5%; P < .01) and normalized plaque volumes (8.0 ± 22.8mm3; P < .01). This resulted in a significant difference in plaque burden between scaffolded and nonintervened segments (P = .03).
ConclusionsIn this small series, the Absorb bioresorbable vascular scaffold showed the potential to provide an additional benefit to pharmacological therapy in locally reducing progression of percent plaque burden. These findings need to be confirmed in larger studies.
Keywords
The clinical introduction of bioresorbable vascular scaffolds (BVS) was the fourth revolution in interventional cardiology. These devices have the unique ability to provide a temporary scaffold, which is necessary to maintain the patency of the vessel after intervention and they gradually permit the restoration of vascular physiology and integrity.1–3 Among the potential advantages of BVS, the reduction in atherosclerotic plaque and late lumen enlargement in the treated regions3–5 may represent a paradigm shift in the treatment of coronary artery disease.
Pharmacological therapy has shown that, depending on the patient's clinical profile, it is possible to promote plaque regression.6–8 Therefore, plaque regression in patients treated with BVS may not be related to the device itself but rather to the effect of pharmacological therapy in a vessel that is free from its internal cage.
The aim of the present study was to perform a within-patient comparison of the natural history of coronary atherosclerosis between segments treated with the poly-L-lactide-acid everolimus-eluting BVS (Absorb BVS first generation, Abbott Vascular; Santa Clara, California, United States) and nonintervened segments in the Absorb Cohort A trial assessed by multislice computed tomography (MSCT).
METHODSStudy PopulationThe design of the Absorb Cohort A trial has been previously described.9 Briefly, it is a single-arm, prospective, open-label study, with safety and imaging endpoints. A total of 30 patients were enrolled at 4 participating sites between March and July 2006. Patients were older than 18 years with a diagnosis of stable, unstable, or silent ischemia. All treated lesions (diameter stenosis > 50%) were single, de novo in a native coronary artery of 3.0mm in diameter, and suitable for the 12- or 18-mm scaffold. Major exclusion criteria were patients presenting with an acute myocardial infarction, unstable arrhythmias, or a left ventricular ejection fraction < 30%, restenotic lesions, lesions located in the left main coronary artery, lesions involving a side branch > 2mm in diameter, and the presence of thrombus or another clinically significant stenosis in the target vessel. The protocol was approved by the ethics committees of the participating institutions, and the enrolled patients gave written informed consent before inclusion. Clinical endpoints were assessed at 30 days, 6 and 9 months, and 1, 2, 3, 4, and 5 years. Noninvasive MSCT imaging studies were done at 18 months and at 5 years of follow-up.
Study DeviceThe study device has been described elsewhere.9 Briefly, the polymeric device consists of a backbone of poly-L-lactide-acid coated with poly-D-L-lactide acid that contains and controls the release of the antiproliferative drug everolimus. Absorb BVS first generation has a crossing profile of 1.4mm in circumferential hoops of poly-L-lactide-acid with struts 150μm thick either directly joined or linked by straight bridges. Both ends of the scaffold have 2 adjacent radio-opaque metal markers. The doses of everolimus on the Absorb BVS 1.0 are 98μg for a 12mm scaffold and 153μg for the 18mm scaffold.
Multislice Computed Tomography AngiographyThe computed tomography (CT) scanners used were 64-slice CT (Brilliance 64, Philips; Best, The Netherlands; and CVi, GE Healthcare; Milwaukee, Wisconsin, United States), 256-slice CT (iCT, Philips), and 320-slice CT.
Computed tomography (Aquilion One, Toshiba; Nasu, Japan), 64-slice dualsource CT (Definition, Siemens AG; Forchheim, Germany), and 128-slice dual-source CT (Definition Flash, Siemens) were used. Standard acquisition techniques were used, which included beta-blockers in patients with a fast heart rate, tube settings depending on patient size (80kV to 140kV), and axial scan protocols for patients with lower heart rates to reduce radiation doses, all at the discretion of the individual sites. Images were reconstructed using thin slices (0.5mm to 0.67mm) and medium smooth reconstruction filters, including 1 or more phases of cardiac cycle, depending on the scan protocol.
Multislice Computed Tomography AnalysisThe MSCT analysis followed a previously established methodology.3,10–12 All datasets were transferred to an offline workstation for analysis using semi-automated plaque analysis software (QAngioCT Research Edition version 2.1, Medis Medical Imaging Systems B.V.; Leiden, The Netherlands). The assessment of the inner lumen and outer vessel volumes was performed following a stepwise approach. First, a centreline originating from the ostium was automatically extracted. Straightened multiplanar reformatted images were generated, and the lumen and vessel borders were detected longitudinally in 4 different vessel views by the software. Cross-sectional images of these longitudinal contours were examined at 0.5-mm intervals and, if necessary, adjusted by an experienced observer. The settings for window level and width were fixed at 740 HU and 220 HU, respectively. Gradient magnitude images, which display the degree of CT attenuation change, were used to facilitate the detection of lumen and vessel wall borders.
Only the major epicardial vessels were considered for analysis using the modified 17-segment American Heart Association model for coronary segment classification (proximal and mid segments of the right, left circumflex and left descending anterior coronary arteries).13 The scaffolded regions were delimited by the presence of the radiopaque markers. If the metallic stents overlapped (n = 3), the scaffolded regions were assessed up to the regions without stent interference. The present study used the intra-patient nonintervened native coronary vessels as comparator for the scaffolded regions by assessing the first 2 proximal segments, divided in proximal or distal according to established anatomical references (Figure 1).13
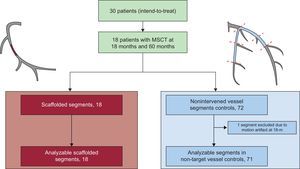
Flowchart of the 5-year serial multislice computed tomography study. Scaffolded segments (in red) were matched at the 18-month and 5-year follow-up for serial comparison. Nonintervened segments delimited by anatomical markers (in blue) were matched at the 18-month and 5-year follow-up for serial comparison. One nonintervened segment had a motion artifact at 18 months, hindering the serial comparison and was excluded. MSCT, multislice computed tomography.
Normalization for segment length provided equal weighting of each patient in the calculation of atheroma volume and also for varying segment length between the 2 scans.10,14 The following intravascular ultrasound-like parameters were calculated for the nonintervened and scaffolded segments after normalization:
Statistical AnalysisContinuous variables are presented as mean ± standard deviation and median [interquartile range, as indicated. Categorical variables are presented as counts and percentages. Continuous variables between the 2 different time points were compared by the paired samples t test. A P value < .05 was considered significant. A K-mean cluster analysis was run using the segment name as a categorical variable and the change in percent atheroma volume as continuous variable (supplementary material). Statistical analyses were performed with use of SPSS version 22.0 software (SPSS Inc.; Chicago, Illinois, United States).
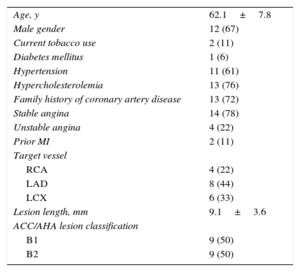
RESULTSPatient demographic characteristics and a flow chart of the present study are shown in Table 1 and Figure 1, respectively. Of the 30 patients enrolled in the Absorb Cohort A trial, 18 underwent serial MSCT at the 18-month and 5-year follow-up and were included in the present analysis. The mean age was 62 ± 8 years, 67% were male, 6% had diabetes mellitus, and 78% had stable angina pectoris. The most frequently treated vessel was the left anterior descending coronary artery (44%) and the mean lesion length at baseline was 9.1 ± 3.6mm.
Baseline Clinical and Angiographic Characteristics (n = 18)
| Age, y | 62.1±7.8 |
| Male gender | 12 (67) |
| Current tobacco use | 2 (11) |
| Diabetes mellitus | 1 (6) |
| Hypertension | 11 (61) |
| Hypercholesterolemia | 13 (76) |
| Family history of coronary artery disease | 13 (72) |
| Stable angina | 14 (78) |
| Unstable angina | 4 (22) |
| Prior MI | 2 (11) |
| Target vessel | |
| RCA | 4 (22) |
| LAD | 8 (44) |
| LCX | 6 (33) |
| Lesion length, mm | 9.1±3.6 |
| ACC/AHA lesion classification | |
| B1 | 9 (50) |
| B2 | 9 (50) |
ACC/AHA, American College of Cardiology/American Heart Association; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; MI, myocardial infarction; RCA, right coronary artery.
Data are expressed as no. (%) or mean ± standard deviation
All scaffolds (n = 18) could be assessed by MSCT at the 18-month and 5-year follow-up. Regarding the nonintervened segments, of 72 possible analysable segments, 1 segment was excluded at 18 months due to motion artefacts (Figure 1). The mean scaffold length was 11.9 ± 1.9mm and the mean length of the nonintervened segments was 22.6 ± 11.7mm.
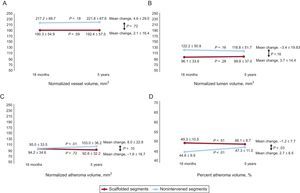
Matched Segment Serial ComparisonBetween the 18-month and 5-year follow-up, scaffolded segments showed no significant change in any of the analyzed parameters, including mean plaque burden, TAV, total lumen volume, and vessel volume (Table 2 and Figure 2). Control segments had a significant temporal increase in atherosclerotic burden as determined by the mean plaque burden (increased in 2.7 ± 6.5%; P = .03) and TAV (increased in 8.0 ± 22.8mm3; P < .01) (Table 2 and Figure 2).
Multisclice Computed Tomography Intravascular Ultrasound-like Analysis Results
| Scaffold (n = 18) | Nonintervened (n = 71) | Scaffold vs non TV* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 18 mo | 5 y | Change | P value | 18 mo | 5 y | Change | P value | ||
| Atheroma volume, % | 49.3±10.5 | 48.1±8.7 | −1.2±7.7 | .51 | 44.6±9.9 | 47.3±11.0 | 2.7±6.5 | < .01 | .03 |
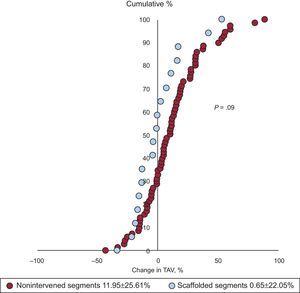
| Change in total atheroma volume, % | 0.6±22.0 | 11.9±25.6 | .09 | ||||||
| Normalized total atheroma volume, mm3 | 94.2±34.6 | 92.6±32.2 | −1.6±18.7 | .72 | 95.0±33.5 | 103.0±36.2 | 8.0±22.8 | < .01 | .10 |
| Normalized total lumen volume, mm3 | 96.1±33.6 | 99.8±37.0 | 3.7±14.4 | .28 | 122.2±50.8 | 118.8±51.7 | −3.4±19.83 | .16 | .16 |
| Normalized vessel volume, mm3 | 190.3±54.9 | 192.4±57.5 | 2.1±16.4 | .59 | 217.2±69.7 | 221.8±67.6 | 4.6±29.0 | .18 | .72 |
TV, target vessel.
Data are expressed as mean [interquartile range] or mean ± standard deviation.
Multislice computed tomography intravascular ultrasound-like parameters. Scaffold segments showed no significant temporal change in vessel, lumen, and plaque volume parameters. The nonintervened segments showed an increase in the plaque volume (C), representing a higher percentage of the vessel area (D).
The change in percent atheroma volume was significantly different between scaffolded regions and nonintervened segments. The mean plaque burden decreased by 1.2 ± 7.7% in the scaffolded segments but increased by 2.7 ± 6.5% in the nonintervened segments (P = .03) (Table 2 and Figure 2). There was also a trend to a difference in the change of normalized TAV (P = .10) and percent change in TAV (P = .09) in favor of the scaffolded segments (Table 2, Figures 2 and 3). The change in the vessel volume was only slightly greater in the nonintervened segments (P = .72). Although the difference between groups was not significant, the larger increase in plaque burden without a proportional increase in vessel volume in the nonintervened segment resulted in an opposite change in the lumen volume; while there was a lumen gain (increase of 3.7 ± 14.4mm3) in the scaffolded segment, lumen loss was observed in nonintervened segments (a decrease of 3.4 ± 19.8mm3; P = .16) (Table 2, Figures 2 and 3). An example of intrapatient comparison of matched segments is given in Figure 4.
Percentage change in the atheroma volume in the scaffolded (blue) and nonintervened vessels (red). Each dot represents one segment. The observed shift to the left in scaffolded regions corresponds to a trend toward atherosclerosis regression compared with nonintervened vessels (P = .09). TAV, total atheroma volume.
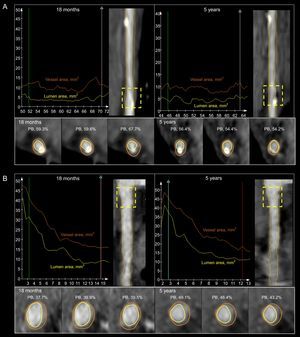
A: lumen and vessel areas of a scaffold implanted in the left anterior descending coronary artery at 18 months (upper panel, left) and 5 years (upper panel, right). There is an increase in the lumen volume and a decrease in the plaque burden (lower panel). B: lumen and vessel areas of the same patient but in the proximal right coronary artery at 18 months (upper panel, left) and 5 years (upper panel, right). There is an increase in the plaque burden (lower panel) and vessel volume with a slight increase in the lumen volume (lower panel). PB, plaque burden.
The main findings of the present study can be summarized as follows: a) Segments treated with Absorb BVS 1.0 showed stabilization of the atherosclerotic process, without a significant paired change in the vessel, lumen, or plaque dimensions; b) nonintervened coronary segments showed a significant increase in the plaque volume and percent atheroma volume, and c) the comparison between scaffolded and nonintervened segments showed a significant benefit from the Absorb BVS scaffold in terms of plaque burden.
Coronary atherosclerosis has posed a challenge to medical practice in terms of reversion of its chronic progressive inflammatory process and subsequent symptoms and events.6,8,10,15,16 In addition, many individual factors and therapeutic interventions may influence coronary plaque modification such as diabetes mellitus, waist circumference, serum CD40L, baseline diastolic blood pressure, sex, and aptitude in improving the lipid profile and C-reactive protein.7,17–19 The present study, being a matched segment within-patient comparison, assessed the long-term progression of atherosclerosis in segments treated by a scaffold and nonintervened segments for the first time. It raises the hypothesis that local therapy with Absorb BVS could provide an additional benefit to pharmacological therapy in terms of atherosclerosis regression. Importantly, the atherosclerosis progression observed in the nonintervened segments is not at variance with previous data that used the same methodology10 and did not result in coronary events.5
The plaque burden reduction in the Absorb BVS-implanted coronary segments has been documented previously.4,20 The explanation for this finding may lie in the ability of mTOR (mammalian target of rapamycin) inhibitors to hinder atherosclerotic plaque formation. Rapamycin and rapalogs are potent inhibitors of vascular smooth muscle cell proliferation. The mTOR inhibitors have antimacrophage properties through different mechanisms such as inhibition of monocyte chemoattractant protein-1 upregulation, impaired recruitment of monocytes to the vessel, and downregulation of de novo protein synthesis.21 The mTOR complex inhibition also prevents lipid accumulation in the plaque due to stimulation of cholesterol efflux and downregulation of low-density lipoprotein and scavenger receptors.21 It has been hypothesized that everolimus may produce a local autophagic response resulting in degradation and/or efflux of lipids via lipophagy and the loss of macrophages in the plaque.22 Indeed, also in animal studies, systemic administration of rapamycin or everolimus has been shown to promote 7% to 85% plaque reduction.21,23,24 However, this process is not fully understood since the Absorb BVS elutes 80% of everolimus within 30 days and the plaque regression in patients treated with Absorb BVS occurs only after 2 years.4,20 We also hypothesize that the disappearance of struts with consequent shrinking of connective tissue may reduce plaque burden.
The impact of 5 coronary devices on plaque sizes by intravascular ultrasound have been compared previously: (Absorb BVS eluting scaffold vs Absorb BVS 1.0 and 1.1; everolimus eluting metallic stent vs Xience V; bare metal stent vs Vision, and paclitaxel-eluting metallic stent vs Taxus).20 At 6 months of follow-up, all devices induced an increase in the total plaque area but Vision and Taxus induced larger increases than the other devices (Absorb BVS [1.0 and 1.1] and Xience V), (P = .0002). The comparison at the 2-year follow-up showed that Absorb BVS 1.1 had a larger increase from post-procedure in total plaque compared with Absorb BVS 1.0, Xience V and Taxus (P = .0499). However, in Absorb BVS 1.1, total plaque showed a reduction of 2.2% from 1 to 3 years. Taxus showed a 9% increase in vessel area, which was much larger than that of Absorb BVS, Xience V or Vision. In addition, Haude et al25 have shown that the DREAMS (drug-eluting absorbable magnesium scaffold) showed a vessel area reduction at 6 months and even more between 6 months and 12 months. These observations highlight the fact that the vessel wall response varies according to the device design. At this point, it is not possible to fully understand whether it is the drug, the polymer, or the constituents of the back bone (metal vs polymer) that play the most determinant role in triggering these changes. However, permanent devices hinder any further reduction of plaque size by lastingly staying in the vessel wall. On the other hand, bioresorbable scaffolds are designed to provide temporary scaffolding of the coronary vessel wall, effectively inhibit neointima formation (by eluting everolimus), and also prevent late complications such as stent thrombosis by their disappearance.
In addition to the plaque burden reduction, it has been hypothesized that Absorb BVS may seal thin-cap fibroatheromas, which are lipid core plaques covered by a thin fibrous cap (< 65μm).26 An optical coherence tomography study has shown that 1 year after Absorb BVS implantation there is formation of symmetric neo-tissue with a mean thickness of 220μm.26 As the device is completely degraded, this may therefore favor the use of a bioresorbable device for the treatment of thin-cap fibroatheromas. Furthermore, preclinical studies have demonstrated that the main component of the neointima following Absorb BVS implantation is fibrous tissue, whereas fibrin and granulomatous cells are infrequent at long-term follow-up.27
Finally, the present study documents the longest noninvasive assessment after Absorb BVS implantation and demonstrates the feasibility of MSCT in following up patients with bioresorbable polymeric devices and quantifying the atherosclerotic burden throughout the coronary tree.
LimitationsThe present study is a retrospective analysis that assessed patients in a first-in-human trial, including patients with low clinical and anatomical complexity. Our results should be considered as hypothesis-generating, given the small sample size herewith described, which does not allow a definitive statement that Absorb BVS should be used as a standard therapy for plaque regression. Additionally, progression/regression studies have shown that that the larger the percent atheroma volume at baseline, the higher the chance of regression. This finding may have the potential to influence the more pronounced regression at scaffolded segments. The ongoing Multicentre Prospective Natural History Study Using Multimodality Imaging in Patients With Acute Coronary Syndromes (PROSPECT ABSORB trial; ClinicalTrials.gov Identifier: NCT02171065) will examine whether the treatment of lesions with plaque burden ≥ 70% with the Absorb BVS plus optimal medical therapy safely increases the minimal lumen diameter at 2 years compared with optimal medical treatment alone and may add further evidence in this regard.
CONCLUSIONSIn this small series, Absorb BVS showed the potential to provide an additional benefit to pharmacological therapy in locally reducing the progression of the percent plaque burden. These findings should be confirmed in larger studies.
FUNDINGThe Absorb Cohort A trial was sponsored by Abbott Vascular (Santa Clara, California, United States).
CONFLICTS OF INTERESTS. Veldhof is full-time employee of Abbott Vascular, Diegem, Belgium.