Keywords
INTRODUCTION
Chagas´ disease is an endemic chronic parasitosis in Central and South America, though its advance into areas of the United States has also been noted due to the increasing population of Latin American immigrants.1,2
Chronic Chagas´ cardiomyopathy (CCM), causes conduction disturbances, ventricular dilatation, ventricular arrhythmias, and sudden death,3,4 and constitutes the most frequent cause of death in many endemic areas.3,5
Patients with CCM have the anatomic substrate for the development of reentrant ventricular tachycardia,6 and have a high incidence of ventricular arrhythmias.4,7 In this setting, early-coupled premature ventricular complexes (PVCs) or the R-on-T phenomenon are frequent findings and are suspected to be the triggers for ventricular tachycardia,4 however, the mode of initiation of spontaneous monomorphic ventricular tachycardia (SMVT) in CCM have not yet been investigated. Third-generation implantable cardiac defibrillators (ICD) can store intracardiac electrograms which are useful for arrhythmia diagnosis and may be used to better understand ventricular tachycardia initiation. The purpose of this study was to examine the mode of initiation of SMVT using stored intracardiac electrograms in this group of patients.
METHODS
Patients
This was a retrospective study that included 22 patients with CCM who underwent ICD implantation since 1998. No patients had symptoms suggestive of coronary artery disease or history of myocardial infarction. Ischemic heart disease was ruled out by a negative stress testing or coronary angiography before ICD implantation in all patients. CCM diagnosis was confirmed by 2 out of 3 specific serologic tests positive for Chagas' disease (enzyme-linked immunosorbent assay, indirect immunofluorescence reaction and indirect hemagglutination) in all patients.
Devices
Seven different types of ICDs were implanted in the study patients (ICD models 1746, 1782, 1810, 1821, 1831, 1851, and 1861; Guidant Corp., St. Paul, MN, USA). The electrograms were recorded between the 2 coil electrodes, which were typically located at the apex of the right ventricle and at the right atrium. Two zones of fast heart rate detection and therapy were programmed in all patients. A ventricular fibrillation zone (upper heart rate) was set with a cut-off rate to detect a ventricular rhythm with a cycle length between 280 and 300 ms and lasting 1 s. A ventricular tachycardia zone (lower heart rate) was programmed with a detection cycle length between 370 and 410 ms, while a zone of slow heart rate was programmed below 40 bpm in all patients, except for 2 patients in whom it was programmed below 50 bpm. A SMVT detection was based on ventricular electrogram rate, morphology, and stability, and the first post pacing interval variability. The pattern of ventricular electrogram was also compared with a sinus rhythm template. In addition, a pattern analysis of atrial and ventricular electrogram relationships, was used in dual chamber ICDs. All stored episodes suggesting SMVT in the intracardiac electrograms which were successfully terminated by the device and which were preceded and followed by at least 7 and 10 electrograms of baseline rhythm, respectively, were analyzed. Upper limits of the first 50 consecutive SMVT events per patient were used for this analysis. PVCs before tachycardia were classified by morphology and by number as single, couplets, and multiple. If the first beat of the tachycardia had the same morphology of the ensuing tachycardia this was considered as single, unless considerable variations of the SMVT cycle length (>10%) at the initiation of the tachycardia were present. In this latter situation, the beats related with longer cycle lengths were denominated couplets or multiple PVCs. Prematurity was evaluated by the coupling interval and a prematurity ratio (PR). A SMVT was considered to be preceded by a "long-coupled" PVC if the PR 30.5 and by a "short-coupled" PVC if the PR <0.5.8 In addition, the presence of short-long-short (SLS) sequences was also evaluated in all episodes.
Data Collection
Follow-up visits were scheduled for the first month following discharge and then every 6 months. Clinical and ICD data were obtained by a cardiologist at each visit. All episodes were stored on floppy disks, which was also done by the cardiologist at each visit. All episodes were stored and reviewed by 2 independent observers. Consensus about the mode of initiation was obtained in cases of discrepancy.
Definitions
- SMVT: sudden change in rate and electrogram morphology from the baseline rhythm with stable cycle length that does not fluctuate by >10% and unvarying electrogram morphology during tachycardia, except for the first 4 beats
- Sudden onset: first beat of the SMVT with the same morphology of the ensuing tachycardia with cycle length variations £10% at the initiation (4 initial beats) of the tachycardia and without preceding premature ventricular complexes
- Extrasystolic onset: SMVT triggered by PVCs with different morphology to the ensuing tachycardia or by PVCs with similar morphology to the SMVT with variations >10% of the SMVT cycle length at the initiation (4 initial beats) of the tachycardia
- Prematurity ratio: coupling interval of the PVC initiating a SMVT divided by the immediately preceding cycle length9
- Short-long-short sequence: interruption of the baseline rhythm by a PVC (short) followed by a pause (coupling interval >1.2 times the previous coupling interval, and by another PVC "short" initiating the SMVT)8
Statistical Analysis
Continuous variables were expressed as mean (SD) or as median and interquartile range, according to the appropriate distribution type and the confidence interval. Statistical significance was assumed if the null hypothesis could be rejected at the 0.05 probability level. All reported P values are 2-tailed. The data were compared with the use of 2-sample t test for continuous variables and c2 test or Fisher's exact tests for categorical variables as appropriate. Because of the number of SMVT episodes differs among subjects, an specific statistical analysis was also performed using the generalized estimating equations (GEE) technique, which consider the varying number of observations that were obtained from each patient.
RESULTS
Clinical Characteristics and Arrhythmic Events
Fourteen out of 22 recipients of third generation ICD devices had at least 1 spontaneous episode of SMVT. The mean age of the patients in our study was 56 (12) years. Mean ejection fraction was 46% (11%) (95% CI, 40.2-51.7). Clinical characteristics of the patients are listed in Table 1. All patients were on sinus rhythm and at the time of SMVT detection, 4 patients were on beta-blockers treatment, while 10 patients were treated concurrently with amiodarone to reduce the number of therapies. The mean dose amiodarone was 300 (170) mg (95% CI, 195-405).
A total of 672 episodes of SMVT were recorded and 222 events of them met the inclusion criteria and 450 were excluded due to a higher number of episodes than the methodological limit of the first 50 episodes in an individual patient (322), lack of electrogram recording (66), scarce baseline rhythm (47), or polymorphic appearance (15).
Mode of Onset of Ventricular Tachycardia
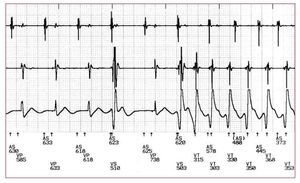
A sudden onset was the most frequent pattern of SMVT initiation (129 episodes, 58%) followed by an extrasystolic initiation (93 episodes, 42%; Figure 1A). Among the latter, 45 (48%), 36 (39%), and 12 (13%) episodes were due to single, couplets and multiple PVCs, respectively. The morphology of the PVC was different than the ensuing SMVT in 88 episodes (95%) of the extrasystolic group. The arrhythmia was preceded by a SLS sequence in 95/222 episodes (43%) in both the extrasystolic and the sudden onset groups (Table 1 and Figure 1A). All SLS sequences were due to a PVC causing a short interval followed by a sinus beat in 65 (68%) or by a paced beat in 26 (32%) and then another PVC (extrasystolic group) or the first beat of the SMVT (sudden onset) producing the second short interval (Figure 2). A comparison between arrhythmia initiation by SLS sequences and other forms of initiation is shown in Table 2.
Figure 1. Graphic representation of the generalized estimated equations (GEE) used to compare proportion of sudden onset and extrasystolic initiation of ventricular taquicardia (VT) and presence or no of short-long-short (SLS) sequences at VT initiation (A) with the confidence intervals and the mean of the variables VT cycle length "VTCL" (B), sinus rhythm cycle length "SRCL" (C), and prematurity index (D), according to (A) categories. B and C values are expressed in milliseconds.
Figure. 2. Electrograms recorded by a cardioverter defibrillator during sustained monomorphic ventricular tachycardia. The tracing shows a ventricular tachycardia onset preceded by a short-long-short sequence.
The mean sinus rhythm cycle length of the previous 7 beats before SMVT onset was 737 (139) ms. Thirty six (16%) episodes were triggered at a sinus rhythm cycle length <600 ms. Only 5 (2%) episodes were triggered at a cycle length >1000 ms. The mean number of morphologies of SMVT per patient was 2.3 (1.2). The mean cycle length of SMVT was 342 (52) ms. When classified according to the mode of initiation, the mean cycle length for SMVT with sudden versus extrasystolic onset were significantly different (347 [60] ms and 333 [36] ms, respectively; P=.05), without differences in the mean sinus rate (731 [138] ms and 745 [140] ms, respectively; P=.47). In addition, the GEE analysis of the variables is exposed in Figure 1B and 1C.
Thirteen of 14 patients (93%) had at least 1 episode initiated by a SLS sequence. Among the 11 patients with more than 1 event, 9 (82%) had SMVT triggered for either sudden or extrasystolic onset, whereas 2 (16%) had a single pattern of initiation during all episodes.
Degree of Prematurity
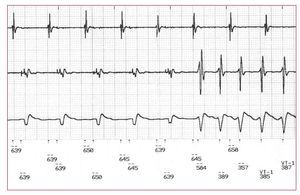
The overall mean coupling interval of the initiating beat of SMVT was 570 (119) ms with a mean PR of 0.72 (0.15). Individual patient data are summarized in Table 1. Two hundred and nine episodes (94%) were initiated by late-coupled PVCs (Figure 3). In 10 (77%) of the 13 episodes triggered by a short coupled interval, the initial beat of the SMVT was preceded by a long cycle length in a SLS sequence, and not to an actual short coupling PVC. The mean long interval was 915 (235) ms. Differences between episodes related or not to a SLS sequence are shown in Table 2. When classified according to the mode of initiation, the mean PR of PVCs triggering a sudden onset (0.67 [0.13]) was significantly lower compared with the mean PR of PVCs that triggered an extrasystolic initiation (0.77 [0.15]; P<.0001). Additionally, a GEE analysis of these variables is shown in Figure 1D.
Figure 3. Electrograms recorded by a cardioverter defibrillator during sustained monomorphic ventricular tachycardia. The tracing shows a sudden onset with a typically long-coupled interval.
DISCUSSION
The main finding of this study is that SMVT is typically initiated by late-coupled PVCs in almost all ICD recipients with CCM. It is generally accepted that SMVT in patients with CCM is due to reentry.6 This model is based on an appropriately timed trigger (PVC) that interacts with a substrate, generating a conduction delay and a circular movement around a fixed obstacle. Patients with CCM have the anatomic substrate for the development of reentrant SMVT.6 We also know that PVCs are frequent findings in patients with CCM and that a number of such patients have multiform PVCs and R on T phenomenon.4 Although there are no data about the mode of SMVT initiation in patients with CCM, R on T phenomenon might be suspected to be the trigger of SMVT. However, short-coupled PVCs were seen exceptionally in the present study. This was also the case in some previous reports in patients with ICDs without CCM, which demonstrated that the onset of SMVT is generally preceded by a late-coupled interval.8,10-12 In this regards, Roelke et al10 reported that among 22 postinfarction patients, the onset of SMVT was preceded by a late-coupled PVC in 86% of the episodes. Other authors revealed that the onset of SMVT was characterized by the presence of a mean PR >0.70 (range, 0.70-0.80) in a general population with ICD.8,11,12
We also found that a sudden onset was the most common form (58%) of SMVT initiation, followed by an extrasystolic onset. This is in contrast with a report in a general ICD population, which revealed a sudden onset of SMVT in 28% of the episodes and an extrasystolic initiation in 66% of the events.12 In that study, a sudden onset appeared to be more common among patients with relatively preserved systolic function. As the mean ejection fraction in our study was higher than that of Saeed et al´s experience (46% vs 29%, respectively), this could partially explain the differences, although this issue warrants further investigation. Furthermore, the SMVT cycle lengths in episodes with sudden onset were longer than in extrasystolic onset in our study. This results are concordant with those reported by Saeed et al,12 who found that the mean cycle length for SMVT with a sudden-onset initiation pattern was 383 (97) ms compared with 336 (88) ms with extrasystolic initiation (P<.002).
Although the findings in this observational study cannot be used to make mechanistic theories, several inferences can be made about the possible direct mechanisms of SMVT initiation. In this regards, a sudden onset could be the result of a concealed slow conduction of the previous sinus beat through the affected tissue emerging at the exit site as the first beat of the SMVT. On the other side, an extrasystolic onset with a different morphology than the ensuing SMVT could be due to focal activity originated in a different site and entering the circuit to start reentry or, similar to the sudden onset initiation, be the result of a previous sinus beat which, after traversing the slow conduction zone, exits the circuit at a different site than the first beat of SMVT. In addition, differences in the modes of initiation of SMVT compared with other substrates could be related to an epicardial origin, which is common in Chagas cardiomyopathy.13
Interestingly, SMVT is also frequently associated (43 percent) with SLS sequences at the time of tachycardia initiation in this study. The reported frequency of SLS intervals triggering SMVT ranged between 2% to 29% in a non-selected ICD population8,12,14,15 Although this pattern of ventricular tachycardia onset is often related with polymorphic ventricular tachycardia, it can also facilitate the induction of SMVT, especially a macroreentry within the His-Purkinje system, favoured by an increased dispersion of refractoriness between adjacent sites.16 The frequent onset of SMVT preceded by a SLS sequence could have therapeutic implications. It can be speculated that programming rate smoothing algorithms to avoid pauses might prevent a substantial number of episodes preceded by a SLS sequence, although 2 previous studies evaluating the utility of antibradycardia pacing algorithms in a general ICD population showed controversial results.17-19 Another potential implication relates to the facilitation of ventricular tachycardia induction by a programmed ventricular stimulation protocol including abrupt changes in ventricular cycle length, which has not been employed heretofore.20 This deserves further investigation in this specific group of patients.
Limitations
This retrospective and observational cohort study included only ICD recipients with SMVT and CCM. Consequently, conclusions should not be extended to other types of ventricular tachycardia or to the whole ICD population.
The small number of patients and the exclusion of a high number of electrograms due to multiple events in the same patient and limited device storage constitute another limitation. However, the great preponderance of late-coupled PVCs as triggers of SMVT and the high prevalence of SLS sequences in almost all patients support the conclusions of the study.
The effect of antiarrhythmic drugs (10/14 patients) and potential differences between patients with normal and depressed ejection fraction are other potential factors that could have influenced arrhythmia initiation and warrant further investigation.
Finally, another important limitation is the likelihood that the arrhythmia was not monomorphic or ventricular in origin, since the interpretation of electrograms was based on recordings between the electrodes used for defibrillation. Severe analysis of local electrograms was not performed, so it is possible that beats apparently similar in fact originated in other parts of the ventricle, as electrograms with different morphology could represent fusion beats with an actual similar site of origin.
CONCLUSION
In this selected group of patients with CCM treated with an ICD, SMVTs are typically initiated by late-coupled PVCs. In addition, the onset of SMVT is frequently preceded by a SLS sequence.
ACKNOWLEDGEMENTS
The investigators wish to thank biostatistical assistance by Xavier Navarro, Erik Cobos, Francesc Miras, and Medtronic Ibérica.
ABBREVIATIONS
CCM: chronic Chagas' cardiomyopathy
ICD: implantable cardioverter-defibrillator
PR: prematurity ratio
PVC: premature ventricular complex
SLS: short-long-short
SMVT: sustained monomorphic ventricular tachycardia
Correspondence:
Dr. M. Abello.
Montañeses 2325. (C1428AQK) Buenos Aires. Argentina.
E-mail: mabello@fleni.org.ar
Received July 2, 2007
Accepted for publication November 27, 2007.