Keywords
INTRODUCTION
Anemia at admission is a frequent finding in patients with acute coronary syndrome (ACS), and has been observed in up to 15% of the patients with myocardial infarction, reaching 43% in elderly patients.1,2 Anemia can adversely influence prognosis in these patients by various mechanisms; on the one hand, by reducing oxygen in the blood and, on the other, by increasing myocardial oxygen consumption due to elevated cardiac output to maintain appropriate tissue oxygenation.3-5
Numerous studies have shown the prognostic value of anemia in this context,2,6,7 and several have also demonstrated that anemia associated with bleeding complications has a poor prognosis,8-11 but few have specifically studied the impact of the fall in admission hemoglobin levels, regardless of bleeding complications.12 Thus, the aim of the study was to determine, together with admission hemoglobin levels, the prognostic value of falls in hemoglobin levels during stay in the coronary care unit.
METHODS
This was a retrospective cohort study that included 542 consecutive patients who were admitted to the coronary care unit for ACS during 2005. It included patients with ACS with persistent ST-segment elevation and patients with ACS without high-risk ST-segment elevation, mainly due to presenting ST-segment abnormalities, mobilized biomarkers of myocardial necrosis or refractory angina.13 Patients with ST-segment elevation ACS were managed by primary angioplasty, whereas patients with non-ST-segment elevation ACS underwent coronary angiography in the first 48 h in most cases. Patients admitted with ACS for coronary angiography transferred from other hospitals were excluded as well as patients who required mechanical ventilation before admission to the coronary care unit (these were admitted to an intensive cardiovascular care unit). Similarly, patients who underwent events in the first 24 h were excluded, with the aim of establishing a predictor linking the fall in hemoglobin levels and the onset of adverse events.
Different clinical and epidemiological variables were prospectively recorded in the unit database, such as age, sex, diabetes, hypertension, dyslipidemia, smoking, a history of ischemic heart disease (stable angina being treated, a background of myocardial infarction, unstable angina, or previous revascularization), a history of previous bleeding, and Killip-Kimball class at admission.14 Other variables were echocardiographic left ventricular ejection fraction (LVEF) in the first 24 hours, the number of main vessels affected as visualized by coronary angiography, the type of revascularization used (angioplasty or surgery), antithrombotic treatment, the need for red blood cell transfusion, and bleeding complications meeting TIMI criteria,15 without including bleeding events in the period following coronary revascularization surgery.
Laboratory Parameters
Hemoglobin levels were measured at admission and at least every 24 hours up to discharge from the unit, while excluding levels following coronary revascularization surgery and patients whose levels were not measured at least twice in the first 24 h. Anemia was defined in line with the criteria of the World Health Organization (hemoglobin <13 g/dL in men and <12 g/dL in women)16 and the fall in hemoglobin levels was calculated as the difference between admission hemoglobin level and the lowest hemoglobin level recorded during this period. Anemia during admission was defined as that which occurred during unit stay in patients who did not meet baseline criteria for anemia. Biomarkers of myocardial necrosis were recorded, mainly troponin-I (normal <0.2 ng/mL), and renal function markers, estimating creatinine clearance using the Cockcroft-Gault formula.17,18
Definition of Events and Follow-up
The main outcome variable was the combination of the following: a) allcause mortality; and b) cardiogenic shock, defined by systolic pressure <90 mm Hg for at least 30 minutes or the need for vasoactive support to maintain systolic pressure >90 mm Hg with signs of bad peripheral perfusion (cold limbs or oligoanuria),19 during hospital admission.
Statistical Analysis
Continuous variables were expressed as average and standard deviation if they followed a normal distribution and as median and interquartile range otherwise. Variables with a nonnormal distribution were compared using the nonparametric Mann-Whitney U test and those with a normal distribution were computed using the Student t test. Discrete variables were expressed as absolute and relative frequencies and presented as percentages and were compared using the c2 or Fisher's exact test. Changes in the proportion of anemic patients were assessed using McNemar's test of exact symmetry. Finally, a binary logistic regression analysis was performed to assess the effect of hemoglobin at admission and the fall in hemoglobin on allcause mortality or cardiogenic shock during the ACS hospital phase. All variables considered to be of clinical relevance (those which other studies had shown to be predictors of events and that were significantly and asymmetrically distributed in our sample) were analyzed as well as those significantly associated with adverse events in the univariate analysis. To avoid overadjusting the model, an intermediate analysis was conducted to determine which variables modified the raw odds ratio (OR) by at least 10% for the fall in hemoglobin or admission hemoglobin levels. The variables that fulfilled this criterion (change in OR >10%) were finally included in the logistic regression analysis. All tests were 2-tailed and a P value less than .05 was considered significant. The SPSS software program version 13.0 (Chicago, Illinois, USA) was used in the statistical analysis.
RESULTS
The baseline characteristics and changes in hemoglobin levels of the patients are shown in Table 1.
At admission, 147 (27.1%) patients presented anemia and this increased to 266 (49.1%) during hospitalization (P<.0001). The median fall in hemoglobin during admission was 0.8 g/dL (interquartile range, 1.6 g/dL).
The patients with anemia at admission were older (72 vs 63 years; P<.001), more women (43.5% vs 24.1%; P<.001), had a history of previous bleeding (19% vs 11.1%; P=.015), diabetic (42.5% vs 25.3%; P<.001), and hypertensive (67.3% vs 51.9%; P<.001), but fewer were smokers (29.3% vs 46.3%; P<.001). In this group, the prevalence of non-ST-segment elevation ACS was higher (60.5% vs 46.6%; P=.004), admission Killip class was worse (Killip ≥II) (20.4% vs 8.1%; P<.001), there was lower creatinine clearance (57.8 vs 77 mL/min; P<.001), less elevated biomarkers of necrosis (35.04 vs 54.6 ng/ mL; P=.03), but more extensive coronary disease (51.7% vs 41.5%; P=.023). On the other hand, the use of antithrombotics was more restricted, and dual antiplatelet therapy (57.1% vs 80.5%; P<.001), platelet GPIIb/IIIa receptor inhibitors (38.8% vs 55.6%; P<.001), and coronary angioplasty (58.2% vs 77.2%; P<.001) were used less.
The patients who developed anemia during admission presented some clinical characteristics and required therapeutic management similar to those of the patients who presented anemia at admission. We highlight the fact that the cohort which developed anemia were older (71 vs 60 years; P<.001), more women (42.1% vs 17.4%; P<.001), worse Killip class (≥II) at admission (17.3% vs 5.1%; P<.001), lower creatinine clearance (62.3% vs 82.5 mL/min; P<.001), and a greater incidence of bleeding complications (8.3% vs 1.1%; P<.001).
During inhospital follow-up, which had a median time of 7 (interquartile range, 5) days, 37 (6.9%) patients presented cardiogenic shock or died. In total, 20 (3.7%) allcause deaths were recorded; 3 patients died in the first 24 hours and 4 presented cardiogenic shock, none of whom were included in the analysis, as mentioned.
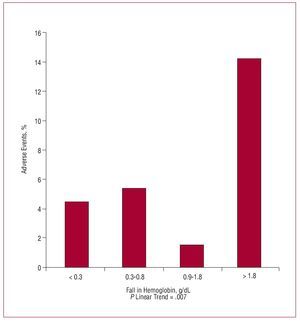
The univariate analysis showed an association between anemia at admission or developing anemia during admission and the incidence of adverse events (Table 2). Similarly, we note that in the patients who presented cardiogenic shock or died the admission hemoglobin levels were lower (12.8 vs 13.9 g/dL; P=.001) and the fall in hemoglobin more marked (2.2 vs 0.8 g/dL; P=.006). Given the lack of linearity between the fall in hemoglobin levels and the incidence of events, the fall in hemoglobin was introduced into the multivariate analysis decomposed into quartiles. The univariate analysis showed a linear trend between fall in hemoglobin quartiles and the incidence of events (P=.007) (Figure).
Figure. Bar chart showing the incidence of adverse events by the fall in hemoglobin in quartiles for all patients.
Other variables associated with worse hospital outcome are shown in Table 3. Possible confounding factors were assessed, including age, sex, the type of ACS, diabetes mellitus, hypertension, smoking, antithrombotic treatment or the type of revascularization, and all the variables significantly associated with the development of adverse events. Finally, the variables demonstrated as modifying the OR of hemoglobin events at admission or the fall in hemoglobin, according to the preestablished criterion, were as follows: Killip class ≥II at admission, LVEF, maximum troponin I level, and coronary disease in more than one main vessel. After adjusting the multivariate analysis for these prognostic variables, the admission hemoglobin levels and the upper quartile of the fall in hemoglobin were independently associated with cardiogenic shock or allcause mortality, with an OR of 5.4 (95% CI, 1.5-18.8; P=.009) for the fall in hemoglobin and 1.4 (95% CI, 1.1-1.8 for each 1 g/dL below normal concentrations according to World Health Organization criteria; P=.003) for the admission hemoglobin levels (Table 4).
In the total sample the incidence of major bleeding complications, according to the TIMI criteria, was 1.1% (6) and minor bleeding complications 3.7% (20). The patients who suffered some bleeding complication presented a greater incidence of adverse events (16.7% vs 6%), OR=3.12 (95% CI, 1.00-9.68; P=.049). However, the presence or absence of bleeding complications barely modified the OR of the fall in hemoglobin or of the admission hemoglobin levels in the intermediate analysis. On the other hand, 12 (2.2%) patients received at least 1 transfusion of red blood cells. The patients who required transfusion had worse clinical outcome (Table 3) but, as in the previous case, the transfusion hardly modified the OR of the study variables.
Finally, the patients who presented anemia at admission and who had a history of previous bleeding received milder antithrombotic treatment, with less use of dual antiplatelet therapy (50% vs 75.5%; P=.003) and platelet GPIIb/IIIa receptor inhibitors (32.1% vs 51.8%; P=.046). Furthermore, they underwent coronary angioplasty less often (50% vs 73.2%; P=.007).
DISCUSSION
Our results show that during hospitalization the prevalence of anemia increased by 55.2%. There is no doubt that the main cause of anemia was blood loss; however, the bleeding complications recorded cannot by themselves account for the increase in the proportion of anemia observed. Both minor bleeding—not sufficiently severe as to be considered as complications—and occult bleeding could indeed explain this change, plus a pathophysiological substratum that would contribute to anemia. Thus, we note that the patients who suffered anemia during admission presented worse Killip class at admission, which suggests that hemodilution could promote the development of anemia.12 Similarly, there were more women and elderly patients. Among these patients, the prevalence of hematopoietic disorder or another is more frequent and would clearly contribute to anemia.15 Kidney failure would also play a key role. Finally, inflammatory reactions in response to myocardial injury could be an additional mechanism, as proinflammatory cytokines are known to inhibit the production and response of erythropoietin and lead to iron metabolism disorders.20,21
Furthermore, the patients who experienced a fall in hemoglobin >1.8 g/dL presented a greater incidence of events which was maintained even after adjusting the analysis by other predictor variables. In this regard, the possible harmful effects of a modest fall in hemoglobin would be counteracted by the benefit of the reduction in blood viscosity,8 but greater falls lead to an increase in myocardial ischemia and the neurohormonal reaction associated with the sudden onset of anemia might determine the worse prognosis observed.3
Several works have studied the prognostic value of anemia associated with evident bleeding or with large falls in levels.8-11 In a recent study of patients who had suffered myocardial infarction, Aronson et al3 showed that for each 1 g/dL fall in admission hemoglobin level, the risk of all cause mortality of heart failure after 2 years increased proportionally and independently.
On the other hand, it has been reported that the admission hemoglobin level is an independent predictor in patients with ACS. Thus, in a large study of elderly patients and myocardial infarction, greater 30-day mortality was associated with lower admission hematocrit levels.2 Similarly, in another retrospective study of 1841 patients, it was found that patients with severe anemia at admission presented greater 30-day mortality.7 In a study with 39 922 patients, Sabatine et al demonstrated a bimodal association between admission hemoglobin levels and worse short-term outcome.6 In this regard, our study did not find such an association between hemoglobin levels and adverse events, although we consider that the sample size did not have sufficient power to detect this.
The poor prognosis associated with anemia at admission would not only be limited to the short term, but would also be maintained in the long term, despite there being some controversy in this regard.22-25 Similarly, anemia would have an unfavorable prognostic impact on patients with stable coronary heart disease.6
As the results indicate, anemia at admission, especially when accompanied by a history of bleeding, restricted the use of intense antithrombotic treatment and favored a more conservative strategy in these patients. However, the possible difficulty arising in this regard was resolved after adjusting the prognostic effects of admission hemoglobin levels for the antithrombotic and revascularization therapy given.
Although our study did not demonstrate transfusion as an independent predictor of events, data in this regard are contradictory. For example, Rao et al observed that in patients with ACS and anemia, transfusion was associated with an increase in 30-day mortality, but there was no impact when hematocrit was less than 25%.15 However, in elderly patients with ACS, Wu et al2 found that transfusion was beneficial when hematocrit was <30%. In the study by Sabatine et al,6 prognosis improved in patients with ACS and ST-segment elevation when transfusion was indicated and hemoglobin levels were <12 g/dL, but worsened when the hemoglobin levels were higher and in patients with ACS without ST-segment elevation regardless of hemoglobin level. Among the mechanisms involved in the potentially adverse effect of transfusion are the depletion of 2,3-diphosphoglyceric acid and nitric oxide stored in red blood cells, with deterioration in tissue oxygen release by hemoglobin, in addition to increasing current endothelial dysfunction.24
In any case, further studies are needed to shed light on this controversy.
The results of the study should be interpreted with the caution reserved for retrospective studies involving less control over patient selection and a lower data quality. Another relevant limitation of our study was the low incidence of adverse events compared to other registries.27
It should be taken into account that events occurring during the first 24 h of admission were not recorded nor were patients who required invasive mechanical ventilation at admission. This limited the capacity of the study to simultaneously introduce all the possible confounding factors. Finally, we do not provide information on the influence of the causes of anemia at admission on adverse events and this remains a pending issue that may modify our knowledge concerning its prognostic value.
CONCLUSIONS
In patients with ACS, anemia at admission is a prevalent comorbidity that increases during admission; the admission hemoglobin levels have an inverse relationship to the incidence of adverse events. Similarly, a fall in hemoglobin >1.8 g/dL was predictor of adverse events regardless of bleeding complications recorded. In view of this, our data indicate that both parameters should be incorporated in the prognostic stratification of patients with ACS.
ABBREVIATIONS
ACS: acute coronary syndrome
CI: confidence interval
GFR: glomerular filtration rate
GPIIb/IIIa: glycoprotein IIb/IIIa
LVEF: left ventricular ejection fraction
OR: odds ratio
Correspondence:
Dr. J.J. González Ferrer.
Instituto Cardiovascular. Hospital Clínico San Carlos.
Prof. Martín Lagos, s/n. 28040 Madrid. España.
E-mail: jjgferrer11@yahoo.es
Received December 26, 2007.
Accepted for publication May 6, 2008.