CYP2C19*2 and CYP2C19*17 alleles appear to contribute to heterogeneous clopidogrel metabolism. The aims of the present study were to assess the phenotype-genotype relationship of CYP2C19*2 and *17 allele carriage and to explore the clinical impact of those polymorphisms at 6-month follow-up of an acute event in an unselected population of non-ST elevation acute coronary syndrome.
MethodsRecruitment for the first and second objectives was 40 stable acute coronary syndrome patients under dual antiplatelet therapy at 12 months after coronary stent placement and an unselected population of 493 consecutive patients with non-ST elevation acute coronary syndrome, respectively. Platelet reactivity was assessed by optical aggregometry induced by adenosine diphosphate and thrombin receptor activating peptide, and by vasodilator-stimulated phosphoprotein phosphorylation measurement using flow cytometry. Genotypes were determined with a TaqMan assay.
ResultsOnly the vasodilator-stimulated phosphoprotein phosphorylation measurement detected significant differences in on-clopidogrel platelet reactivity between the wild-type subjects and the CYP2C19*2 (P=.020) and *17 allele carriers (P=.048). No significant difference was found between CYP2C19*2 ([HR (95%CI): 1 (0.94-1.55)], P=.984) or *17 ([HR (95%CI): 0.93 (0.61-1.43)], P=.753) allele carriage and the occurrence of adverse events at 6-month follow-up.
ConclusionsEven though CYP2C19 genotype is associated with variable on-clopidogrel platelet reactivity, it has no significant clinical influence. Prognosis of acute coronary syndromes may be influenced by a myriad of variables.
Keywords
Clopidogrel is a prodrug that must be metabolized to exert its irreversible inhibitory effect on the P2Y12 adenosine diphosphate (ADP) receptor. Clopidogrel metabolization into the thiol active compound requires two oxidative steps involving hepatic cytochrome P450 (CYP) isoenzymes, such as CYP2C19, 3A4/5, 1A2, 2B6, and 2C9.1 Some studies suggest that CYP polymorphisms may be responsible, at least in part, for heterogeneous response to clopidogrel.2 Recently, the in vitro contribution of each CYP was analyzed, demonstrating that clopidogrel metabolism mainly depends on CYP2C19.1 Thus, loss-of-function alleles of CYP2C19, such as *2 allele, is associated with impaired conversion of clopidogrel to its active metabolite, with superior on-clopidogrel platelet reactivity and poor outcomes, particularly increased risk of stent thrombosis.3, 4, 5 On the other hand, the CYP2C19*17 allele associates with enhanced platelet response to clopidogrel,6 and carriers of this polymorphic variant might benefit more from clopidogrel treatment than do noncarriers7. Yet, the clinical significance of these CYP2C19*2 and *17 remains unclear; recent analyses in other series of patients provide differing results regarding the relationship between clopidogrel efficacy and allele carrier status.3, 4, 5, 6, 7
Our aims were: to assess the phenotype-genotype relationship according to the CYP2C19*2 and *17 status in 40 stable patients under dual antiplatelet therapy by using different platelet function methods, and to explore the prognostic influence of those polymorphisms in an unselected cohort of 493 non-ST segment elevation acute coronary syndrome (nSTE-ACS) patients at 6 months of follow-up.
METHODS PatientsFor the first objective, we included 40 stable Caucasian patients under both acetylsalicylic acid and clopidogrel therapy; 12 months before their inclusion, all study participants had undergone percutaneous coronary intervention (PCI) for recurrent events after stent implantation. Inclusion criteria were age older than 18 years, absence of any hematological disorder or contraindication to dual antiplatelet therapy, and absence of ischemic events requiring hospitalization for at least 6 months before inclusion in the study. Clinical and demographic characteristics as well as details of the PCI were recorded from medical records. Bare-metal or drug-eluting stents were implanted, following current recommendations, at the discretion of the interventional cardiologist. After intervention, 100mg/day of acetylsalicylic acid and 75mg/day maintenance dose of clopidogrel were continued in keeping with current protocols.8
For the second objective, we enrolled 493 consecutive Caucasian patients diagnosed with nSTE-ACS and admitted to one of 3 participating tertiary hospitals in Spain; however, 65 patients were excluded due to early discontinuation of the clopidogrel therapy. Finally, 428 patients were followed for 6 months. We included patients who presented at the Emergency Department with at least 2 of the following criteria: a) typical chest pain; b) electrocardiographic changes of ischemia, such as down-sloping ST segment changes ≥0.5mm in at least 2 consecutive leads or T inversion ≥0.2mV, and c) elevation of troponin T levels above the standard cut-off point (≥0.1ng/mL). Patients with concomitant infectious/inflammatory disease, hematological dyscrasia, or contraindications for antiplatelet therapy were excluded. All the patients received standard management as recommended for ACS under current guidelines,8 using initial dual antiplatelet therapy, glycoprotein-IIb/IIIa inhibitors as well as β-blockers, angiotensin-converting enzyme inhibitors, low molecular weight heparin, and statins when appropriate. In addition, TIMI risk score for nSTE-ACS was calculated in all patients.9 Patients subsequently underwent coronary angiography and/or PCI at the discretion of the admitting cardiologist. The 6-month follow-up consisted of outpatient clinic visits, telephone contact, and review of clinical notes. We defined “adverse endpoint” as cardiovascular death or recurrent acute coronary syndrome requiring hospital admission. All patients gave written informed consent and the study was approved by the local ethics committee.
Laboratory ProceduresFor the first objective, blood samples were collected at 8:00 am in the outpatient clinic, in overnight fasting conditions, including abstinence from tobacco and alcoholic or caffeine-containing beverages. At the time of sampling all patients were taking daily dual antiplatelet therapy (100mg acetylsalicylic acid and 75mg clopidogrel). Blood was collected by venipuncture in 3.2% trisodium-citrated tubes for platelet reactivity assays or in ethylenediaminetetraacetic acid (EDTA) tubes for genomic DNA collection. Platelet-rich plasma for aggregation assays was obtained from citrated blood by centrifugation at 150/g for 10min at room temperature and used without platelet count adjustment within 3h after blood collection.
For the second objective, venous blood samples were collected within the first 48h after patient admission, always before coronary angiography, using serum and EDTA tubes. For genomic DNA assays (both objectives) EDTA tubes were centrifuged at 1500/g for 12min at room temperature; the leukocyte strata were collected and stored at −80°C until batch analysis. Troponin-T levels in nSTE-ACS patients were determined in serum samples at admission and at 6 and 12h, using one-step enzyme immunoassay based on electrochemoluminiscence technology (Elecsys, Roche Diagnostics, Basel, Switzerland).
Platelet Function Assays Platelet Aggregation AssaysLight transmittance aggregometry was performed using a standard aggregometer (Aggrecorder II, Menarini Diagnostics, Florence, Italy) set at 37°C and 1000rpm. Changes in light transmission were monitored for 5min in unadjusted platelet-rich plasma10 after stimulating with 5μM and 10μM ADP (DiaMed, Cressier, Switzerland) and 25μM thrombin receptor activating peptide (TRAP) (Sigma-Aldrich Inc, St Louis, Missouri, United States). The endpoint of platelet aggregation corresponds to percentage of maximal change in light transmission, considering the autologous platelet-poor plasma as 100%. The TRAP-inducible aggregation response was used as an intra-assay positive control for each patient, as this response is mediated by the thrombin receptor protease-activating receptor-1 and is not blocked by thienopyridines.
Platelet Reactivity Index-Vasodilator Stimulated Phosphoprotein AssayThe measurement of the vasodilator-stimulated phosphoprotein (VASP) phosphorylation levels is a marker of P2Y12 reactivity and thus of clopidogrel-induced inhibition.11 Phosphorylated VASP levels were quantified through assessment of the platelet reactivity index (PRI) by flow cytometry (Beckman Coulter FC500, Miami, Florida, United States) using labeled monoclonal antibodies and following a standardized assay (Platelet VASP, Diagnostica Stago, Biocytex Inc, Marseille, France) according to manufacturer's instructions. Platelet population was identified on forward and size scatter distributions and 5000 events were gated. The extent of VASP phosphorylation was assessed by difference in the geometric mean fluorescence intensity (MFI) values in samples incubated with PGE1 in the presence or absence of ADP (MFIPGE1+ADP, and MFIPGE1, respectively). After subtraction of negative isotypic control values from the corresponding fluorescence values, PRI(%) was calculated according to the following formula: PRI(%)=[(MFIPGE1)-(MFIPGE1+ADP)/(MFIPGE1)]×100. The ratio was expressed as mean percentage platelet reactivity, and inversely correlated with the clopidogrel-mediated platelet inhibition.
GenotypingGenomic DNA was purified using the Puragene Blood Core Kit B (QIAGEN Sciences, Germantown, Maryland, United States). Genotype analysis of the CYP2C19*2 (681G>A; rs4244285) and CYP2C19*17 (–806C>T; rs12248560) polymorphisms was performed with TaqMan SNP Genotyping Assays (Applied Biosystems, Carlsbad, California, United States) following the manufacturer's instructions, using LC480 Real Time PCR (Roche, Basil, Switzerland). Genotypes were blind-analyzed for the patient's platelet aggregation values and clinical outcome. To control for correct sample handling, genotyping was repeated in 20% of the patients, providing identical results. All patients included in the study were genotyped for both allele variants; thus the “call rate” was 100%.
Statistical AnalysisVariables are presented as mean±standard deviation, counts (percentages), or median (interquartile range). Kolmogorov-Smirnov test was used to check for normal distribution of continuous data. Categorical variables were compared using χ2 test. Comparisons among continuous variables were assessed using two-sided unpaired t-student or Mann Whitney U tests depending on the data distribution. Calculations for endpoints of interest were based on the comparison of the CYP2C19*2 and CYP2C19*17 allele carriage status among wild-type subjects (G/G or C/C, respectively) and carriers of the polymorphic allele (combination of both G/A and A/A, and C/T and T/T, respectively). A dominant model was chosen in both cases because the polymorphic */A or */T alleles result in a complete loss or gain of enzyme function, which means that one mutant allele exerts a relevant effect on overall enzyme activity. Deviation from the Hardy-Weinberg equilibrium was assessed in both populations using χ2 test for each polymorphism. Unadjusted Cox regression proportional hazards model was used to compare outcomes throughout the follow-up period between polymorphic and wild-type allele status, with the wild-type being the reference group in each case. The overall free-survival rates were calculated using Kaplan-Meier method, and the differences determined using the log rank test. All P-values <.05 were accepted as statistically significant. Statistical analysis was performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, Illinois, United States).
RESULTS Relationship Between CYP2C19 Genotype and On-Treatment Platelet ReactivityBaseline clinical characteristics of the 40 patients assessed for on-treatment platelet reactivity are shown in Table 1. Briefly, the mean age in this population was 65.8±10 years; 36 (90%) were males. All patients had stent placement, 32 (80%) of them drug-eluting and 8 (20%) bare-metal stents. The genotype distribution was 77.5% GG, 17.5% GA, and 5% AA for the CYP2C19*2 polymorphism and 67.5% CC, 30% CT, and 2.5% TT for the CYP2C19*17 polymorphism. For the CYP2C19*17 genotype distributions, no significant deviation from the Hardy-Weinberg equilibrium was observed (P=.698), unlike CYP2C19*2 (P=.009).
Table 1. Baseline Characteristics of 40 Non-ST-Segment Elevation Acute Coronary Syndrome Patients Included in 1st Objective.
| Variable | CYP2C19*2 allele | CYP2C19*17 allele | Total | ||
| G/G | */A | C/C | */T | ||
| Patients, no. (%) | 31 (77.5) | 9 (22.5) | 27 (67.5) | 13 (32.5) | 40 (100) |
| Age, years | 65.9±8.9 | 65.6±13.7 | 66.5±10.7 | 64.1±8.6 | 65.8±10 |
| Age ≥65 years, % | 54.8 | 66.7 | 63 | 46.2 | 57.5 |
| Sex (male), % | 93.5 | 77.8 | 85.2 | 100 | 90 |
| Hypertension, % | 78.6 | 66.7 | 68 | 91.7 | 75.7 |
| Dyslipidemia, % | 64.3 | 44.4 | 52 | 75 | 59.5 |
| Diabetes, % | 50 | 33.3 | 44 | 50 | 45.9 |
| Smoking habit, % | 25 | 0 | 16 | 25 | 18.9 |
| BMS, % | 22.6 | 11.1 | 22.2 | 15.4 | 20 |
| DES, % | 77.4 | 88.9 | 77.8 | 84.6 | 80 |
BMS, bare-metal stent; DES, drug-eluting stent.
Age is expressed as mean±standard deviation.
On-treatment platelet reactivity in patients according to CYP2C19*2 and CYP2C19*17 polymorphisms is summarized in Table 2. As shown, nonsignificant differences were observed between wild-type and polymorphic subjects in the residual platelet reactivity induced by 5 and 10μM ADP measured by light transmittance aggregometry. The TRAP-induced aggregation measured by light transmittance aggregometry was in the normal range for healthy subjects among wild-type and polymorphic patients (data not shown).
Table 2. Effect of the CYP2C19 Genotype in On-Clopidogrel Adenosine Diphosphate-Induced Maximal Platelet Aggregation in Patients With Elective Percutaneous Coronary Intervention and Stent Implantation.
| Polymorphism | No. | Platelet aggregation, ADP 5μM | Platelet aggregation, ADP 10μM |
| CYP2C19*2 | |||
| G/G | 31 | 47.1±14.3 | 54.2±15.5 |
| */A | 9 | 54.2±12.5 | 62.6±17.4 |
| P-value | .190 | .170 | |
| CYP2C19*17 | |||
| C/C | 27 | 50.3±14.6 | 58.1±16.4 |
| */T | 13 | 45.5±12.8 | 51.9±15.4 |
| P-value | .319 | .265 |
ADP, adenosine diphosphate.
Results are shown as mean values of maximal percentage of aggregation, mean±standard deviation.
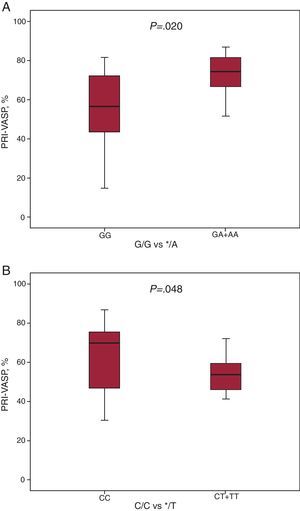
On the other hand, CYP2C19*2 */A allele carriers displayed higher platelet reactivity in the PRI-VASP than wild-type patients (G/G) (72.3%±11.9% vs 56.1%±18.8%, P=.02) (Figure 1A). Likewise, lower PRI-VASP was significantly found among CYP2C19*17 */T allele carriers compared with wild-type (C/C) patients (51.3%±17.8% vs 63.8%±18%, P=.048) (Figure 1B).
Figure 1. Effect of the CYP2C19 genotype on platelet reactivity index-vasodilator-stimulated phosphoprotein assay on patients that underwent elective percutaneous coronary intervention and stent implantation. A, effect of the CYP2C19*2 allele variant. B, effect of the CYP2C19*17 allele variant. Vasodilator-stimulated phosphoprotein phosphorylation levels were evaluated by flow cytometry in citrated whole blood by mean of a commercially available assay as described in “Methods”. Boxes describe interquartile range. Solid line is median, and lower and upper limits denote the 2nd and 98th percentiles. PRI-VASP, platelet reactivity index-vasodilators-stimulated phosphoprotein.
We analyzed patients at high risk of thrombosis according to the cut-off level for on-clopidogrel platelet reactivity in the setting of PCI proposed in a recent consensus.12 In our population, 26 (65%) and 29 (72.5%) patients displayed high on-treatment platelet reactivity as measured by ADP-induced aggregation and PRI-VASP, respectively. Despite the low number of patients evaluated, both methods used to test residual platelet reactivity showed a trend for higher representation of patients with increased on-clopidogrel platelet reactivity among carriers of the CYP2C19*2 */A allele, whereas carriage of the CYP2C19*17 */T allele did the opposite (Table 3).
Table 3. Effect of CYP2C19*2 and CYP2C19*17 Genotype on the Prevalence of High-Risk Patients According to High On-Clopidogrel Platelet Reactivity.
| Polymorphism | Patients, no. (%) | LTA-ADP (5μM) ≥46%, no. (%) | PRI-VASP ≥50%, no. (%) | |
| CYP2C19*2 | CYP2C19*17 | |||
| A | ||||
| G/G | - | 31 (77.5) | 18 (58.1) | 20 (64.5) |
| */A | - | 9 (22.5) | 8 (88.9) | 9 (100) |
| P-value | .091 | .036 | ||
| - | C/C | 27 (67.5) | 20 (74.1) | 22 (81.5) |
| - | */T | 13 (32.5) | 6 (46.2) | 7 (53.9) |
| P-value | .084 | .074 | ||
| B | ||||
| G/G | C/C | 19 (47.5) | 13 (68.4) | 14 (73.7) |
| */A | C/C | 8 (20) | 7 (87.5) | 8 (100) |
| G/G | */T | 12 (30) | 5 (41.7) | 6 (50) |
| */A | */T | 1 (2.5) | 1 (100) | 1 (100) |
| Total | 26 (65) | 29 (72.5) | ||
LTA-ADP, maximal platelet aggregation induced with adenosine diphosphate; PRI-VASP, platelet reactivity index-vasodilator-stimulated phosphoprotein.
The effects of each polymorphic allele carriage on platelet function tests has been assessed in two ways: A) individual effect of each polymorphism, and B) combination of both polymorphisms.
Data are shown as number of cases (percentage of cases). Patients included in 1st objective were classified as “high-risk patients” when maximal aggregation induced by ADP 5μM was ≥46%, or when PRI-VASP values were ≥50% according to cut-offs established by Bonello et al. 12 in a recent consensus. Prevalence of high-risk patients among carriers and noncarriers of the variant alleles were compared by using χ2 test.
Because of early discontinuation of clopidogrel therapy in 65 included cases, 428 patients were finally recommended by their cardiologist and assessed in our study. Demographic and basal characteristics of the 428 patients are detailed in Table 4. The mean age was 67.3±12.2 years in the cohort of 278 (65%) males and 150 (35%) females. Concerning nSTE-ACS presentation, 39.6% of enrolled patients presented down-looping ST segment and 59.3% showed increased Troponin-T levels (above standard cut-off point). Mean TIMI risk score of the entire cohort was 3.22±1.54. The genotype distribution was 71.96% G/G, 24.77% G/A, and 3.27% A/A for the CYP2C19*2 polymorphism, and 66.12% C/C, 30.14% C/T, and 3.74% T/T for the CYP2C19*17. For genotype distribution, no significant deviation from the Hardy-Weinberg equilibrium was observed (P=.527 and P=.905, respectively). Thus, we found 120 patients with the presence of the CYP2C19*2 */A allele (28%) in our population, and 145 CYP2C19*17 */T allele carriers (33.9%) (Table 4). As shown, there were nonsignificant differences (P>.05) in baseline and demographic characteristics, medications, and management of patients irrespective of genotype (Table 4), with the exception of significant differences in down-looping of ST segment among CYP2C19*17 allele carriers (P=.05) as well as catheterization (without statistical significance in stent placement rates) and nitrate prescription frequencies (P=.034 and .037, respectively).
Table 4. Baseline Characteristics of Patients With Non-ST-Segment Elevation Acute Coronary Syndrome Enrolled to Perform the 2nd Objective, According to CYP2C19 Genotype.
| Variable | CYP2C19*2 | CYP2C19*17 | Total | ||
| G/G | */A | C/C | */T | ||
| Patients, no. (%) | 308 (72) | 120 (28) | 283 (66.1) | 145 (33.9) | 428 (100) |
| Age, years | 67.3±12 | 67.3±12.8 | 67.3±12.2 | 67.3±12.2 | 67.3±12.2 |
| Age ≥65 years, % | 65.6 | 58.3 | 65 | 60.7 | 63.6 |
| Sex (male), % | 64.3 | 66.7 | 66.4 | 62.1 | 65 |
| Hypertension, % | 68.1 | 75 | 68.8 | 72.4 | 70 |
| Dyslipidemia, % | 55.2 | 53.3 | 54.8 | 54.5 | 54.7 |
| Diabetes, % | 42.7 | 41.7 | 40.1 | 46.9 | 42.4 |
| Smoking habit, % | 22.4 | 21 | 23.4 | 19.3 | 22 |
| At least 3 CVRF, % | 39.2 | 40 | 37.7 | 42.8 | 39.4 |
| ST segment descent, % | 37.8 | 44.2 | 42.9 | 33.1 | 39.6 |
| Troponin T ≥0.1 ng/mL, % | 59.2 | 59.8 | 60.6 | 56.9 | 59.3 |
| Significant lesion, % | 30.2 | 31.3 | 29.9 | 31.7 | 30.5 |
| Symptoms of severe angina, % | 29.4 | 37.4 | 29.2 | 36.4 | 31.6 |
| TIMI score | 3.2±1.5 | 3.4±1.6 | 3.2±1.5 | 3.2±1.6 | 3.2±1.5 |
| Management during hospitalization | |||||
| Catheterization | 71.3 | 75.2 | 75.7 | 66 | 72.4 |
| With stent | 46 | 44.6 | 48.3 | 40.7 | 45.6 |
| Without stent | 25.3 | 30.6 | 27.4 | 25.3 | 26.8 |
| CABG | 10.1 | 14.4 | 11.7 | 10.4 | 11.2 |
| Treatment during 6-month follow-up period | |||||
| ASA | 96.4 | 95.8 | 95.4 | 97.9 | 96.3 |
| Clopidogrel | 100 | 100 | 100 | 100 | 100 |
| β-blockers | 86.8 | 90.9 | 90 | 83.3 | 87.9 |
| ACEI | 55.3 | 50 | 56.9 | 46.9 | 53.7 |
| Nitrate | 28.1 | 39.6 | 36 | 21 | 31 |
| ARB | 14.2 | 22.7 | 18 | 13.5 | 16.6 |
| CCB | 20.1 | 20.4 | 22.3 | 15.6 | 20.2 |
| Statin | 89.5 | 90.9 | 92 | 85.4 | 89.9 |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; CABG: coronary artery by-pass grafting; CCB, calcium channel blockers; CVRF, cardiovascular risk factors; significant lesion, coronary stenosis ≥50%.
Age and TIMI score expressed as mean±standard deviation.
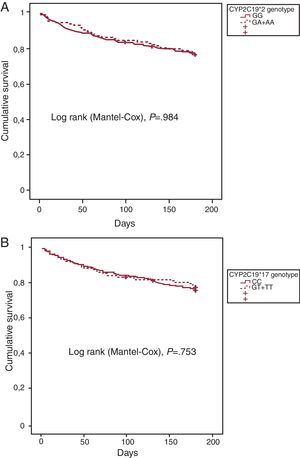
Complete 6-month follow-up data were available in 412 (96.3%) of the 428 patients assessed. Among those, 97 (23.5%) patients had adverse events in this follow-up period. In univariate Cox regression analysis (Table 5), we found that CYP2C19*2 loss-of-function allele (*/A) carriage was not a significant predictor of adverse outcomes ([HR (95%CI): 0.99 (0.64-1.55)], P=.984). Moreover, gain-of-function allele CYP2C19*17 (*T) was not significantly associated with lower endpoint rates at 6-month follow-up ([HR (95%CI): 0.75 (0.61-1.43)], P=.753). As illustrated in Figure 2, cumulative survival at this short follow-up period was not significantly influenced by the CYP2C19 genotype, according to the univariate analysis (Table 5). Conversely, several of the risk factors used to calculate the TIMI risk score showed statistically significant impact on clinical outcome in the univariate analysis, such as age ≥65 years, electrocardiographic abnormalities with ST segment descent, and symptoms of severe angina (Table 5). Indeed, the TIMI risk score was a potent predictor of adverse clinical outcomes in the whole population ([HR (95%CI): 1.41 (1.23-1.62)], P<.001). Additionally, female sex was significantly associated with adverse clinical outcome ([HR (95%CI): 1.62 (1.09-2.42)], P=.018). Nonetheless, in multivariate analysis only age ≥65 years and electrocardiographic abnormalities with ST segment descent remained significantly associated with adverse clinical outcomes at 6-month follow-up (P=.001, and P=.028, respectively) (Table 5). A trend towards poor outcome was shown for symptoms of severe angina during the preceding 24h (P=.051).
Table 5. Cox Regression Analysis for the Endpoint Events (Yes/No) Followed up During 6 Months According to Genotype and TIMI Risk Score Factors (2nd Objective).
| Variable | HR (95%CI) | P-value |
| Univariate analysis | ||
| CYP2C19*2; */A vs G/G | 1 (0.94-1.55) | .984 |
| CYP2C19*17; */T vs C/C | 0.93 (0.61-1.43) | .753 |
| Female sex | 1.62 (1.09-2.42) | .018 |
| Age ≥65 years | 2.45 (1.50-4.01) | <.001 |
| Cardiovascular risk factors ≥3 | 1.28 (0.86-1.91) | .225 |
| Coronary stenosis ≥50% | 1.40 (0.91-2.10) | .132 |
| ST segment depression | 1.68 (1.13-2.51) | .011 |
| Previous ASA | 1.40 (0.92-2.10) | .113 |
| Symptoms of severe angina | 1.60 (1.04-2.50) | .034 |
| Troponin T ≥0.1ng/mL | 1.34 (0.87-2.05) | .182 |
| TIMI risk score | 1.41 (1.23-1.62) | <.001 |
| Multivariate analysis | ||
| Female sex | 1.29 (0.82-2.02) | .272 |
| Age ≥65 years | 2.52 (1.44-4.42) | .001 |
| Coronary stenosis ≥50% | 1.47 (0.88-2.46) | .138 |
| ST segment depression | 1.63 (1.05-2.53) | .028 |
| Previous ASA | 1.10 (0.66-1.82) | .722 |
| Symptoms of severe angina | 1.56 (1-2.42) | .051 |
ASA, acetylsalicylic acid; 95%CI, 95% confidence interval; HR, hazard ratio.
The reference group used to calculate the interaction between underlying allele status for *2 and *17 in the univariate analysis was the wild-type allele variant.
Figure 2. Kaplan Meier curves for CYP2C19 *2, and *17 allele carriers and non-carriers followed up during 6 months. A, cumulative survival for CYP2C19*2 allele carriers (GA+AA, discontinuous line) and noncarriers (GG, continuous line). B, cumulative survival for CYP2C19*17 allele carriers (CT+TT, discontinuous line) and noncarriers (CC, continuous line).
DISCUSSIONDiscrepancies reported in on-clopidogrel platelet reactivity and the impact of both polymorphisms, CYP2C19*2 and *17, may be related to the number of patients tested, but also to the fact that the PRI-VASP test specifically explores the P2Y12 receptor function and seems to be more sensitive to test biological effects of clopidogrel according to the CYP2C19 status (Table 3), unlike the P2Y receptors driving ADP-induced platelet aggregation13 by optical aggregometry. In addition, the poor correlation between the various platelet function tests14, 15 appears to vary according to the underlying influencing factors for high on-treatment platelet reactivity.16
Several trials have found an association of CYP2C19*2 and poor clinical prognosis.3, 4, 17 Our data suggest that the clinical impact of a single polymorphism may have been reduced in these moderate-risk patients because fewer than 50% of them underwent PC7, 18 for stent placement.18 Other studies have found that ischemic events and stent thrombosis occurred early after starting clopidogrel treatment in association with PCI and stent placement procedures.3, 4, 18, 19 Patients undergoing PCI may have a different status of platelet reactivity.20 Indeed, several studies have found positive results when more than 70% of patients underwent PCI3, 4, 5, 19 irrespective of the small sample size.2, 19 On the contrary, in a genetic analysis of the Clopidogrel in Unstable Angina to prevent Recurrent Events (CURE)7 trial, when lower PCI frequencies were assessed (18% PCI, 15.5% with stent), CYP2C19*2 polymorphic allele status (*2/*2, *2/*3 and *3/*3) was not associated with adverse events.
Diabetes has been consistently associated with platelet hyperreactivity, and together with CYP2C19*2 polymorphism constitutes the best discriminating factor to explain insufficient response to clopidogrel.21 Nonetheless, results from a recent meta-analysis in which 91% of the patients had undergone PCI found that the impact of CYP2C19*2 allele status was more associated with high risk of adverse clinical outcomes than other clinical factors.5 According to those findings, diabetes was more frequent (42.4%) in our population than others (23%-28%).5, 18 However, higher diabetes prevalence in our population is apparently not sufficient to significantly increase the risk of *2 allele carriers, even when combined with a PCI frequency of nearly 70% or a stent implantation rate of almost 50%. Therefore, PCI and stent placement may be considered as high risk factors, more so than other clinical factors, in CYP2C19*2 allele carriers under dual antiplatelet therapy. Accordingly, “high risk populations” must be defined with care depending on the underlying clinical scenarios. Even then, CYP2C19*2 allele status only explains 5% to 15.2% of the heterogeneous response to clopidogrel.2
On the other hand, the clinical significance of CYP2C19*17 allele carriage remains to be fully elucidated. Some studies have related this polymorphism with greater response to clopidogrel7 and an increased risk of bleeding.18, 22 There is little agreement about a neutral6 or protective effect on ischemic events.22, 23 Our results failed to find a significant interaction between *17 allele status and adverse events, even when lower rates of catheterization were reported in *17 allele carriers than noncarriers (66% vs 75.7%, P=.034), probably due to our moderate-risk population. Positive studies associating CYP2C19*17 with a reduction of adverse events are based on a reduced number of patients undergoing PCI (18%)7 and stent deployment (15.5%). Nevertheless, higher PCI rates (90%-100%) are associated with nonreduction in ischemic endpoints, irrespective of CYP2C19*17 allele status.4, 6 The PLATO trial might be comparable with ours, given the PCI frequencies (66%), but it provides information only about the association between *17 allele status and bleeding risk.18 Thus, in patients with moderate to higher thrombotic risk the “protective” clinical impact of CYP2C19*17 may be lower. Unfortunately, we did not record bleeding rates in our patients to explore the influence of these polymorphisms in possible hemorrhagic complications4, 6, 7, 18 in our particular cohort.
The high interindividual variability of the response to clopidogrel remains a clinically relevant issue.24, 25, 26, 27 Even though such heterogeneity has been shown to be a multifaceted process,24, 25, 28, 29 several studies have suggested a link among CYP2C19 genotype and prognostic implication.30, 31, 32 However, female sex (35%) was consistent with adverse clinical outcomes, in concordance with results from Mega et al.5 The individual prognostic relevance may depend on the clinical setting.21, 33 Demographic and basal characteristics, together with CYP2C19 status,19, 21, 33, 34 in our cohort (42.2% diabetes, 35% female sex, and 45.6% stent placement) may explain, at least in part, the trend (albeit nonsignificant) of the Kaplan Meier survival curves. A phenotype results from the combination of genotype and the environmental influence that may be the most important variable in cardiovascular disease.21, 33
LimitationsFirst, we found a high incidence of high-residual platelet reactivity among first objective patients, which may be a limitation to achieve statistical differences according to genotype in platelet function testing by using light transmittance aggregometry. We do not find a categorical explanation for these results. However, a higher rate of DES use related with defective neointimal healing in coronary arteries may be related with higher platelet reactivity.
Second, sample size in both populations was limited and was based on the maximum number of patients consenting to participate. We cannot exclude that it was underpowered, as in previous reports.18 The allelic prevalence was very similar to other studies,3, 4, 5, 6, 7 with 3.3% and 3.7% of A/A (CYP2C19*2) and T/T (CYP2C19*17), respectively. Moreover, we assessed entirely Caucasian-based populations; thus our results might be specific to our patient populations and the way they were managed. Therefore, our study might underestimate the influence of genetic polymorphisms on the effects of clopidogrel in our particular settings. Moreover, major studies assessing the impact of CYP2C19 had longer follow-up periods; we performed a relatively short 6-month follow-up, as previously.5
We studied only the two most common polymorphisms associated with clinical outcomes; however, other alleles or baseline/demographic factors may be relevant for individual patients. Another limitation of the 2nd objective study is the short follow-up period. The lack of assessing clopidogrel compliance in the 2nd objective population also may be a limitation. Finally, the use of two different populations precludes establishing associations between on-treatment platelet reactivity, cardiovascular outcomes, and CYP2C19 allele status.
CONCLUSIONSThe CYP2C19*2 */A and CYP2C19*17 */T allelic variants are significantly associated with increased and diminished on-clopidogrel platelet reactivity, respectively, when the specific effect of clopidogrel on the P2Y12 is tested.
However, CYP2C19 status has inconsistent influence on adverse events in unselected nSTE-ACS patients at moderate risk within a 6-month follow-up. Such populations may be more impacted by other clinical/demographic factors. A different impact may exist in clopidogrel benefit with respect to preventing adverse events such as stent thrombosis or recurrence of acute coronary syndrome. Arterial thrombosis might be influenced by a myriad of variables. Findings obtained by any trial should be interpreted with caution depending on the different clinical settings.
FUNDINGThis study has been supported by Instituto de Salud Carlos III (RECAVA RD 06/0014-0039), Fundación Séneca (07703/GERM/07), and Beca Fundación Uriach-Sociedad Española de Cardiología 2009.
CONFLICT OF INTERESTNone declared.
Received 23 February 2011
Accepted 12 July 2011
Corresponding author: Centro Regional de Hemodonación, Ronda de Garay s/n, 30003 Murcia, Spain. jose.rivera@carm.es