Keywords
INTRODUCTION
The onset of atrial fibrillation (AF) is due, in most cases, to ectopias originating in the pulmonary veins (PV) and, less often, in other atrial structures.1-7 Identifying and locating the ectopic foci is a complex procedure that, in patients without abundant spontaneous ectopic activity, requires the use of various maneuvers aimed at revealing them and mapping the causative foci. Although these procedures initially formed the basis for AF ablation,4,5 they have subsequently been relegated to second place largely due to ignorance regarding how well the induction maneuvers perform and the clinical meaning of the induced arrhythmias because of the lack of systematic studies.
The aim of our study was to analyze: a) the inducibility of atrial arrhythmias via adenosine and isoproterenol infusion in patients with AF, b) the reproducibility of induction, c) locating the causative ectopic foci, and d) the clinical meaning of the induced arrhythmia.
METHODS
A retrospective analysis was made of the results obtained using the pharmacological induction protocol used in our center for identifying ectopic foci in patients with AF referred for ablation. All these patients had paroxysmal or persistent AF refractory to, at least, a class I or III antiarrhythmic drug. Following our clinical protocol, pharmacological induction was used in patients with AF who presented a high density of atrial extrasystoles (>10/h) and/or paroxysmal atrial tachycardia (AT) or AF during Holter monitoring obtained before ablation, but without sufficient spontaneous ectopia at the time of ablation to be able to establish its origin.
Electrophysiological Study and Pharmacological Maneuvers
A 24-electrode catheter (Orbiter®, Bard Electrophysiology) was advanced into the coronary sinus making a loop in the right atrium, thus making it possible to monitor wide zones of the left and right atrium with the distal and proximal electrodes, respectively. By using the double-guide technique via a single transeptal puncture guided by intracardiac sonography,8 a circular decapolar catheter (Lasso®, Biosense Webster) and an ablation catheter with a 4-mm tip (Navistar®, Biosense Webster) were positioned in the proximal segment of the superior PV. The PV were previously visualized via selective angiography and an anatomical reconstruction was done using the CARTO® system (Biosense Webster). The patients with AF at the time of the study underwent cardioversion once the catheters were positioned in the left atrium.
Due to its ease of use and speed of effect, adenosine is used first in our pharmacological induction protocol, and then isoproterenol. In given circumstances (contraindications for adenosine use, induction of repetitive ectopia, induction of AF, presumed predominant sympathetic AF, etc), and depending on the criterion of the acting physician, the order of the drugs could be switched or some of them dispensed with not used.
Adenosine was administered as an ultra-rapid intravenous bolus via the peripheral route at an initial dose of 6 mg, with 6-mg increases in successive boli until reaching a maximum dose of 18 mg or atrial ectopia induction. Patients with bronchial asthma or chronic obstructive pulmonary disease did not receive adenosine. Isoproterenol was administered as continuous infusion beginning with a dose of 1 µg/min, which was increased at a rate of 1 µg/min every 5 min until reaching a maximum dose of 4 µg/min, an increase in baseline sinus rhythm higher than 30%, the induction of ectopia, or the appearance of side effects. The electrophysiological study was done without sedation or general anesthesia, except in the patients who required electro-cardioversion, where a brief period of profound sedation was induced with propofol (1-2 mg/kg).
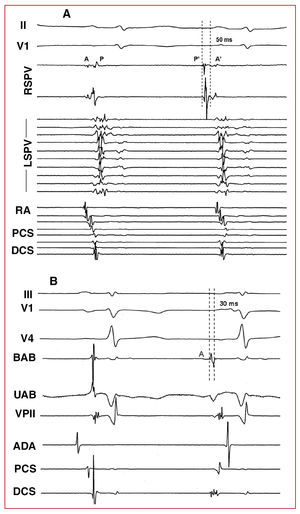
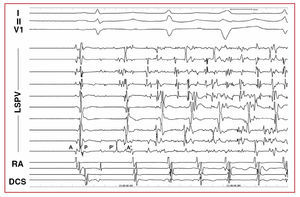
Three types of pharmacological response were distinguished: isolated atrial ectopia, AT, and AF. The location of the focus was established according to the origin of the ectopic beats, estimated via premature local atrial activation higher than 30 ms in respect to the start of the P wave, the analysis of the atrial activation sequence in the coronary sinus and right atrium, the unipolar electrocardiograms and electroanatomic mapping when possible.9,10 When the induced arrhythmia was AF, the location of the focus was estimated via mapping the first ectopic beat that initiated the AF (Figures 1 and 2).
Figure 1. Intracavitary monitoring of ectopia induced in 2 different patients after adenosine administration. A: isolated ectopia proceeding from the PVSD; the first beat corresponds to sinus rhythm and the second to ectopia. Note the inversion of the electrocardiograms in the RSPV, the premature potential of the pulmonary vein (P') with respect to the P wave of the surface electrocardiogram, and the change in sequence in the RA, PCS and DCS recordings. B: ectopia proceeding from the vein of Marshall region. The interval between the bipolar ablation (BAB) catheter electrocardiograms and the P wave is 30 ms. Note the QS-type morphology under unipolar ablation (UAB). II and V1 indicates ECG leads; RA, right atrium; DCS, distal coronary sinus; PCS, proximal coronary sinus; RSPV, right superior pulmonary vein; LSPV, left superior pulmonary vein; A, atrial electrocardiograms in sinus rhythm; P, electrocardiogram of pulmonary vein in sinus rhythm; A', atrial ectopia electrocardiograms; P', pulmonary vein ectopia electrocardiograms.
Figure 2. Initiation of atrial fibrillation from the LSPV. After the first beat (sinus), LSPV ectopia occur that initiate atrial fibrillation. Note the inversion of the electrocardiograms and their prematurity in the LSPV during the first ectopic beat. I, II, V1 indicates ECG leads; RA, right atrium; DCS, distal coronary sinus; LSPV, left superior pulmonary vein; A, atrial electrocardiogram in sinus rhythm; P, electrocardiogram of pulmonary vein in sinus rhythm; A', atrial ectopia electrocardiogram; P', pulmonary vein ectopia electrocardiogram.
Ablation
Once the provocation maneuvers were done radiofrequency ablation was carried out using the CARTO system®. In the cases where ectopia or AF was induced in a consistent and reproducible manner, only this focus was ablated, directly in the case of extrapulmonary foci, or through electric isolation of the ostia in the case of PV foci. Empirical isolation of the PV was done in the cases where reproducible ectopia was not induced. The immediate success of ablation was defined by the disappearance of the ectopia in the case of extrapulmonary foci or the bidirectional blockage of venoatrial conduction when electric isolation of the PV was done. Long-term success was defined by the absence of AF recurrence within a period of 6 months from the time of ablation, evaluated clinically and with serial Holter monitoring in the first, third and, sixth month.
Statistical Analysis
The quantitative variables were adjusted to normal distribution using the Shapiro-Wilk test, and thus are expressed as mean ± standard deviation (SD). They are analyzed using parametric tests (Student t). The qualitative variables are expressed as the number (percentage) of observations and are analyzed using χ², or the Fisher exact test if the expected values are <5. P-values ≤.05 were considered statistically significant.
RESULTS
A total of 53 patients were included in the analysis. The average of age of the population studied was 50±13 years, with more males (37 patients, 70%). There was no structural heart disease in 94% of the patients. The size of the left atrium was 42±6 mm. In total, 46 patients had a history of paroxysmal AF (87%) and 7 (13%) had persistent AF. The average number of antiarrhythmic agents used before ablation was 2.5±0.8.
Of the 53 patients included in the study, 43 (81%) did not present baseline spontaneous ectopia and the ectopia was insufficient in 10 (19%) to establish its origin (mean 12±4 extrasystoles/h), thus indicating the pharmacological induction protocol. The monitoring time prior to administration of the induction agents was 122±24 min (time between the start of baseline electrocardiographic record and the administration of the first drug).
In the pharmacological induction protocol adenosine was used in 21 patients, isoproterenol in 17 patients, and both drugs in 15 patients. No significant differences were found regarding baseline characteristics in each group of patients. During adenosine infusion, the patients described general discomfort, flushes, or dyspnea lasting seconds producing high-grade atrioventricular block in 50, 86, and 100% of the patients when receiving doses of 6, 12 and 18 mg, respectively. During the administration of isoproterenol in doses of ≥2 µg/min, the patients reported palpitations, sometimes associated with sweating. No other adverse effects associated with the administration of adenosine and isoproterenol occurred.
Some atrial arrhythmia was induced in 46 patients (88%) after the administration of adenosine and/or isoproterenol. The rate of inducibility in patients with paroxysmal AF was 87% (40 out of 46 patients) and 86% (6 out of 7 patients; P=NS) in patients with persistent AF. Isolated ectopia was induced in 31 patients (65%), AT in 4 patients (8%) and AF in 13 patients (27%). The latter was sustained and required electro-cardioversion in 10 of the 13 patients (77%). In the patients who presented some baseline ectopia, the P-wave morphology of the induced extrasystoles was similar to that of the spontaneous ones. Tables 1 and 2 show the origin and coupling intervals, respectively, of the first ectopic beat of the induced arrhythmias. In 10 patients (19%), the induced ectopia had an extrapulmonary location.
Adenosine
Thirty-six patients received 54 injections of adenosine. In total, 46 boli of adenosine (85%) induced some arrhythmia in 31 of the 36 patients (86%), which consisted in isolated ectopias in 26 patients (84%), AT in 1 patient (3%) and AF in 4 patients (13%). In 21 adenosine boli (46%), the induction of arrhythmia preceded or was not associated with changes in heart rate, in 8 boli (17%) the arrhythmia appeared at the time of bradycardia, and in 17 boli (37%) the arrhythmia appeared after bradycardia (mean, 15±25 s afterwards). The probability of induction increased in direct proportion to the dose of adenosine used. Doses of 6, 12, and 18 mg yielded a positive response in 4 out of 8 injections (50%), in 33 out of 37 injections (89%) and in 9 out of 9 injections (100%), respectively (P=.02). No significant relationship was found between the dose of adenosine and the type of effect, isolated ectopia, AT, or AF. To assess the reproducibility of the induced ectopia, 18 additional boli of adenosine were administered in 14 patients (maximum of 3 per patient), some ectopia being induced in all cases (of the same type in 10 patients, and of equal type and location as the initial ectopia in 8 patients).
Isoproterenol
Thirty-two patients received isoproterenol infusion. The administration of isoproterenol induced some arrhythmia in 27 patients (82%), which consisted of isolated ectopias in 14 patients (52%), AT in 3 patients (11%) and AF in 10 patients (37%). At the time of induction, the sinus rhythm had not been modified in 3 patients (11%), 7 patients (26%) had experienced an increase in sinus rhythm <30% of baseline, and the remaining 17 patients (63%) had experienced an increase of >30%. Overall, the average increase in sinus rhythm at the time of induction was 27±18%.
Ablation Guided by Inducibility
In 32 of the 46 patients in whom induction was possible (70%), only the induced ectopia was ablated (1.4±0.6 substrates per patient), yielding long-term success in 21 patients (66%). There were no significant differences regarding the results of ablation depending on the type of arrhythmia induced. Nine of the 11 patients with AF recurrence after the first ablation underwent a second procedure. In 4 patients, only the substrate previously treated was ablated due to recurrence of the initial focus, or venoatrial conduction and reproducibility of previous inducibility. Long-term success was achieved in all these patients. Another substrate was ablated in 1 patient with persistent AF during follow-up. Empirical isolation of the 4 PV was done in 4 patients, the AF persisting in 3 patients.
The remaining patients, in whom induction was possible (14 patients), were treated via ablation of the induced ectopia and the remaining PV (4.2±0.7 substrates per patient), achieving long-term success in 13 patients (93%). Overall, the duration of these procedures was 339±55 min.
Figure 3 shows the results of ablation according to the strategy followed and the inducibility of atrial arrhythmias.
Figure 3. Results of AF ablation depending on the pharmacological inducibility of atrial arrhythmias and ablation strategy. In the group of patients in whom induction was possible, ectopic ablation refers to the direct ablation of the focus or to the isolation of a pulmonary vein when this was the origin of the focus. In the group of patients in whom induction was impossible, the low-density spontaneous extrasystoles that the patients had during the electrophysiological study were used when ablation was guided by the ectopia. PV indicates pulmonary vein.
DISCUSSION
The present study presents the results of using 2 pharmacological maneuvers, the infusion of adenosine and isoproterenol, in patients with AF refractory to antiarrhythmic agents referred for ablation, who had abundant ectopia under previous Holter monitoring, but which at the time of the procedure lacked sufficient ectopic density to locate the causal foci of the AF. In general, adenosine and isoproterenol showed a high rate of inducibility of atrial arrhythmias in these patients (86 and 82%, respectively).
Adenosine is a drug widely used in the treatment of supraventricular tachycardia. Its mechanism of action is mainly on the sinus and atrioventricular nodes as it stimulates the potassium current IK, Ado, Ach, which causes cellular hyperpolarization and consequent interruption of tachycardias dependent on the atrioventricular node.11 The relevance of adenosine for inducing ectopia and/or AF rests on its proarrhythmic effects.12,13 The reported frequency of adenosine-induced AF reaches 12%,14 which is coherent with that found in our study. In the atrium, adenosine shortens action potential duration and refractory periods. Furthermore, it increases the sympathetic tone reflex causing arterial hypo-tension.15,16 By modifying the refractory periods, adenosine promotes the development of functional reentry in susceptible patients.17 Such reentrant activity has a particular predilection for the PV, due to its anisotropic conduction and repolarization heterogeneity.17 Although adenosine directly reduces automatism and pacemaker activity, the increase in sympathetic tone reflex produced after adenosine administration can promote abnormal automatism and pacemaker activity, once the bradycardia period has ended, which was observed in 35% in our series. In our study, the induction of atrial arrhythmias with adenosine presented dose-dependant behavior, and inducibility was more frequent the greater the dose administered.
Isoproterenol is an adrenergic agonist that, through the accumulation of intracellular calcium, promotes an increase in pacemaker activity and abnormal automatism.18,19 Like adenosine, isoproterenol has been used as an inducer of ectopia and/or AF.3,5,20 In our work, the rate of inducibility of atrial arrhythmias with isoproterenol was comparable to that found with adenosine, although there were differences in the type of arrhythmia induced, since isoproterenol induced AF more frequently than adenosine. Most patients who presented a positive response to isoproterenol did so after an increase in sinus rhythm, requiring increases >30% in baseline sinus rhythm in 63% of patients.
The disappearance of AF recurrence after ablation of only the induced ectopia confirmed its pathogenic and clinical importance. In the series studied by Marchlinski et al,6 ablation guided exclusively by the induced ectopia produced a long-term success rate of around 80%, with an average of 3 treated substrata per patient. In our series, the success of ablation guided exclusively by induced ectopia was 66% after treating an average of 1.4 substrata per patient, increasing to 78% after carrying out a second ablation procedure on the same substratum.
It might be thought that a practical application of the use of pharmacological induction maneuvers could be to reduce the number of ablated substrata by identifying the causal foci of the AF in each patient. However, even though induced ectopia has a specific character, ablation exclusively only of the identified foci may leave untreated other ectopic foci not identified by these drugs or important structures which maintain AF, as demonstrated in the first published series of AF ablation.2-5 In our series, patients in whom induced ectopia was treated, and also the remaining PV, had a higher long-term success rate (93%). Thus, we consider that the main usefulness of pharmacological induction maneuvers could be to identify ectopic foci not dealt with via empirical ablation of the 4 PV. Our study shows that, in nearly 20% of the patients undergoing AF ablation who presented previous abundant ectopia, the causal focus of the arrhythmia had an extrapulmonary location. Marchlinski et al6 found an extrapulmonary origin in 16% of patients with AF who received ablation guided by drug-induced ectopia. More recently, Lee et al7 analyzed a series of 293 patients with spontaneous ectopic beats that induced AF during electrophysiological study and found that in 20% of patients the ectopia arose from both PV and extrapulmonary foci, and that only 12% arose from extrapulmonary foci.
CONCLUSIONS
Adenosine and isoproterenol infusion during sinus rhythm frequently induces atrial ectopia in patients with drug-refractory AF and evidence of abundant previous ectopia. The selective ablation of induced ectopia prevents the recurrence of AF in a significant percentage of patients, which confirms its clinical importance. The systematic use of pharmacological induction maneuvers could increase the success of AF ablation, by making it possible to identify foci not dealt with via empirical ablation of the 4 PV.
Limitations
Even though at the time of the electrophysiological study the patients were in sinus rhythm without significant spontaneous ectopia, they presented a high density of ectopia and/or frequent AF episodes under previous Holter monitoring. This indicates that ectopic foci are strongly involved in the pathophysiology of AF in these patients. Thus, the results of this study cannot be extrapolated to patients with AF dependent on other mechanisms. Another limitation is the retrospective nature of the analysis. Obviously, the low number of patients and the absence of randomization regarding the ablation strategy means that no conclusions can be drawn in relation to the relative value of empirical or mixed guided ablation.
Correspondence:
Dr. N. Pérez-Castellano.
Unidad de Arritmias. Hospital Clínico San Carlos. Instituto Cardiovascular.
Prof. Martín Lagos, s/n. 28040 Madrid. España.
E-mail: nperez.hcsc@salud.madrid.org
Received September 5, 2005. Accepted for publication March 9, 2006.