Potassium derangements are frequent among patients with chronic cardiovascular conditions. Studies on the associations between potassium derangements and clinical outcomes have yielded mixed findings, and the implications for health care expenditure are unknown. We assessed the population-based associations between hyperkalemia, hypokalemia and clinical outcomes and health care costs, in patients with chronic heart failure, chronic kidney disease, diabetes mellitus, hypertension, and ischemic heart disease.
MethodsPopulation-based, longitudinal study including up to 36 269 patients from a health care area with at least one of the above-mentioned conditions. We used administrative, hospital and primary care databases. Participants were followed up between 2015 and 2017, were aged ≥ 55 years and had at least 1 potassium measurement. Four analytic designs were used to evaluate prevalent and incident cases and the use of renin-angiotensin-aldosterone system inhibitors.
ResultsHyperkalemia was twice as frequent as hypokalemia. On multivariable-adjusted analyses, hyperkalemia was robustly and significantly associated with an increased risk of all-cause death (HR from Cox regression models ranging from 1.31–1.68) and with an increased odds of a yearly health care expenditure >85th percentile (OR, 1.21–1.29). Associations were even stronger in hypokalemic patients (HR for all-cause death, 1.92–2.60; OR for health care expenditure> percentile 85th, 1.81–1.85).
ConclusionsExperimental studies are needed to confirm whether the prevention of potassium derangements reduces mortality and health care expenditure in these chronic conditions. Until then, our findings provide observational evidence on the potential importance of maintaining normal potassium levels.
Keywords
Chronic cardiovascular, metabolic and renal conditions such as chronic heart failure (CHF), chronic kidney disease (CKD), diabetes mellitus (DM), ischemic heart disease (IHD) and hypertension affect potassium (K+) homeostasis through several mechanisms.1–3 These include deleterious mechanisms inherent to the disorders themselves, as well as those caused by some of their pharmacological therapies.4 All of these can ultimately lead to impaired or excessive K+ excretion. As a consequence, K+ derangements and particularly hyperkalemia are highly prevalent in individuals with these conditions, ranging in different studies from 5.7% in a population with CKD with a follow-up of 18 months to 8.2% in cohorts of patients with acute heart failure.3–6
This is particularly true among users of renin-angiotensin-aldosterone system inhibition (RAASI) therapies,5,6 such as angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), renin inhibitors (RIs), angiotensin II receptor blocker neprilysin inhibitors (ARNIs) and mineralocorticoid-receptor antagonists (MRAs). Despite this common adverse effect, the overall benefits in terms of survival and other outcomes demonstrated in multiple landmark clinical trials make these class I therapies for the treatment of some of these conditions, such as CHF.7–9
Studies on the associations between K+ derangements and clinical outcomes have so far yielded mixed findings. Some observational studies and meta-analyses have reported associations between both hypokalemia and hyperkalemia and an increased risk of all-cause mortality in CHF, CKD, and DM.5,10 Conversely, some recent epidemiological analyses have failed to demonstrate an association between K+ derangements and mortality in CHF patients.11 Of note, analyses on this important research question using large health care databases are currently scarce, as are studies including assessments of the medical resource use and expenditure associated with K+ derangements.
The aim of the present study was to gain a better understanding, from a population-based perspective, of the impact of deranged K+ levels—both hyperkalemia and hypokalemia—on the clinical outcomes, medical resource use and health expenditure of patients with chronic cardiovascular, metabolic and renal conditions (CHF, CKD, DM, hypertension or IHD), in whom RAASI therapies (ACEIs, ARBs, MRAs or RIs) may be indicated.
MethodsData sourcesFor the present analysis, we linked 2 large, population-based, automated health care databases from our public health system. These databases capture complementary, individual-level, longitudinal health-related information of all residents. For the present analysis, one database was used as the source for sociodemographic characteristics, medical conditions (coded using either the International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] coding system for in-hospital diagnoses, the International Classification of Diseases, 10th Revision [ICD-10] for diagnoses generated in primary care settings, and the clinical classifications software for both), and medication dispensing (coded using the anatomical therapeutic chemical coding system). The other database was used to identify incident events during follow-up, as well as for health care resource use and associated health care costs calculations. In addition, we linked these databases to a third, laboratory test result database that captures any laboratory test results generated during routine clinical care in both hospitals and primary care settings. This database has been available since January 1st, 2015.
Setting, study period, and populationWe restricted our analyses to the metropolitan health care area of our hospital, which comprises approximately 1.2 million persons. The study period was defined between January 1st, 2015 and December 31st, 2017. Inclusion criteria were: a) age at study entry ≥ 55 years and b) availability of at least one serum K+ measurement during the evaluation period. Additional criteria were applied for each of the 4 analyses conducted, as detailed below.
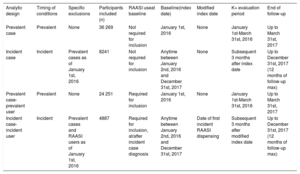
Study designWe used 4 analytic approaches to evaluate the associations between K+ derangements, and the study outcomes under different sets of assumptions: prevalent case, incident case, prevalent case-prevalent (RAASI) user, and incident case-incident (RAASI) user analyses (table 1).
Summary of analytic designs used
| Analytic design | Timing of conditions | Specific exclusions | Participants included (n) | RAASI useat baseline | Baseline(index date) | Modified index date | K+ evaluation period | End of follow-up |
|---|---|---|---|---|---|---|---|---|
| Prevalent case | Prevalent | None | 36 269 | Not required for inclusion | January 1st, 2016 | None | January 1st-March 31st, 2016 | Up to March 31st, 2017 |
| Incident case | Incident | Prevalent cases as of January 1st, 2016 | 8241 | Not required for inclusion | Anytime between January 2nd, 2016 and December 31st, 2017 | None | Subsequent 3 months after index date | Up to December 31st, 2017 (12 months of follow-up max) |
| Prevalent case-prevalent user | Prevalent | None | 24 251 | Required for inclusion | January 1st, 2016 | None | January 1st-March 31st, 2016 | Up to March 31st, 2017 |
| Incident case-incident user | Incident | Prevalent cases and RAASI users as of January 1st, 2016 | 4887 | Required for inclusion, at/after incident case diagnosis | Anytime between January 2nd, 2016 and December 31st, 2017 | Date of first incident RAASI dispensing | Subsequent 3 months after modified index date | Up to December 31st, 2017 (12 months of follow-up max) |
K+, potassium; n, number; RAASI, renin-angiotensin-aldosterone system inhibitor(s).
In the “prevalent case” analysis (figure 1 of the supplementary data), we included individuals with at least 1 prevalent relevant chronic cardiovascular, metabolic or renal condition (CHF, CKD, DM, hypertension, or IHD) as of January 1st, 2016 (regardless of their use of RAASI medications). Evidence of these diagnoses was sought in the database using operational definitions based on ICD-9-CM, ICD-10, and clinical classifications software codes (tables 1-3 of the supplementary data). For each of these individuals, we evaluated any K+ levels recorded in the database between January 1st and March 31st, 2016 (“evaluation period”). Information on other relevant covariates was collected at the end of the evaluation period (ie, March 31st, 2016), except for estimated glomerular filtration rate (eGFR), which was evaluated during the preceding year. Clinical outcomes were assessed between March 31st, 2016 and up to 12 months of follow-up (ie, March 31st, 2017).
In the “incident case” analysis (figure 2 of the supplementary data), included individuals could not have recorded evidence of any of the 5 relevant conditions as of January 1st, 2016, and had a first recording of at least 1 of those conditions between January 2nd, 2016, and December 31st, 2017 (“index date”). K+ levels were evaluated, for each participant, between the index date and the subsequent 90 days (“evaluation period”). Clinical endpoints were assessed for up to 12 months after each patient's evaluation period.
In the “prevalent case-prevalent user” analysis (figure 3 of the supplementary data), participants had to have at least 1 relevant condition as of January 1st, 2016 to be included and to be using at least one RAASI medication (ACEIs, ARBs, MRAs, or RIs) in the preceding 3 months. The use of these medications was identified using operational definitions combining anatomical therapeutic chemical codes (table 4 of the supplementary data). The rest of the analytic design is comparable to that of the prevalent case analysis presented above.
Finally, in the “incident case-incident user” design (figure 4 of the supplementary data), participants could not have recorded evidence of a relevant condition or of RAASI medication use as of January 1st, 2016. The first recording of at least 1 relevant condition had to occur between January 2nd, 2016, and December 31st, 2017 (“index date”), with evidence of subsequent dispensing of at least 1 RAASI medication. The date of the first dispensing of a RAASI drug was the “modified index date”. For each participant, we then evaluated any K+ levels recorded in the database between the modified index date and the subsequent 90 days (“evaluation period”). The rest of the design features are similar to those of the incident case analysis.
Definition of K+ profilesIn each of the 4 analyses, recorded laboratory test results during the evaluation period were reviewed for serum K+ levels. Hyperkalemia was defined as serum K+ levels> 5.0 mEq/L; hypokalemia as serum K+ levels <3.5 mEq/L, and normokalemia as serum K+ levels ≥ 3.5 and ≤ 5 mEq/L. Based on the (1 or more) K+ level measurements available for each participant during the evaluation period, 6 clinical profiles were defined a priori: a) “hypokalemic” patients, those who had evidence of 1 or 2 episodes of hypokalemia (with at least 7 days between the 2 episodes, otherwise they were considered part of the same episode) and no evidence of hyperkalemia; b) “hyperkalemic” patients, those who had 1 or 2 episodes of hyperkalemia during the evaluation period (with at least 7 days between the 2 episodes, otherwise they were considered part of the same episode) and no evidence of hypokalemia; c) “normokalemic” patients, those who had no evidence of hyper or of hypokalemia during the evaluation period; d) “recurrent hyperkalemic” patients, those individuals with 3 or more episodes of hyperkalemia and no hypokalemia; e) “recurrent hypokalemic” patients, those individuals with 3 or more episodes of hypokalemia and no hyperkalemia; and f) “mixed K+ derangements” patients, those with both hyper- and hypokalemic episodes. The very small number of participants in groups d through f (n <5 for all) resulted in their exclusion and analyses were restricted to hyperkalemic, hypokalemic, and normokalemic patients.
Other relevant covariatesInformation on other relevant covariates was collected at the end of the evaluation period, including age, sex, comorbidity index (using the adjusted morbidity groups comorbidity and complexity classification system,12table 5 of the supplementary data), individual income, K+ derangement-associated medications (ACEIs, ARBs, MRAs, RIs—when appropriate—, beta-blockers, K+ supplements, loop diuretics, nonsteroidal anti-inflammatory drugs, trimethoprim, and macrolides [table 4 of the supplementary data for lists of anatomical therapeutic chemical codes]), disease duration, number of prior hospitalizations, number of prior emergency department visits, and number of serum K+ level tests.
Clinical outcomes and health care resource useThe primary outcome of interest was all-cause death. As secondary outcomes, urgent hospitalizations, emergency department visits and hospital daycare visits were also evaluated. All outcomes were identified using the information recorded in the database, which covers all public health care (hospital and primary care) centers.
In an exploratory analysis, yearly total health care expenditure during 2016 was also assessed. These calculations were performed using the methodology applied in prior health care expenditure analyses conducted for patients with chronic cardiovascular conditions.13 Healthcare expenditure was calculated as the expenditure in euros (€) per person per year. This included direct costs such as pharmacy expenditure or billing invoices, which were assigned to each individual patient through their personal health identification number. Indirect expenditure was used for primary care, hospital care and skilled nursing facilities and was weighted by visits by professional (ie, nurse or physician), diagnosis-related groups, and length of stay, respectively. Table 6 of the supplementary data presents average health-related costs in Catalonia as of 2016. The outcome of interest for this analysis was defined as having an overall expenditure> 85th percentile of the distribution for 2016 in our area.
Statistical analysisThe baseline characteristics of the study population included in each of the 4 study designs (prevalent case, incident case, prevalent case-prevalent user, and incident case-incident user) were described at the index date, overall and by K+ profile (hyperkalemic, hypokalemic, normokalemic). Categorical variables were compared across K+ groups using chi-squared tests, and continuous variables using ANOVA and nonparametric tests, as appropriate.
The crude incidence rate of the first occurrence of each study outcome was described during follow-up, per 1000 person-years, for each of the K+ profiles. Kaplan-Meier survivor function curves were also used to graphically describe their event-free survival.
We used Cox proportional hazards regression models to calculate the multivariable-adjusted hazard ratios (HR) of each of the clinical endpoints, comparing hyperkalemic and hypokalemic patients, respectively, to normokalaemic patients (reference group), and adjusting for the following potential confounders: age, sex, adjusted morbidity groups, medication use, individual income, and median eGFR levels during the preceding year. In prevalent analyses, we further adjusted for disease duration, number of hospitalizations, number of prior emergency department visits, and number of serum K+ level tests (all in the 6 months before January 1st, 2016). For endpoints other than mortality, Fine and Gray models were used to account for the competing risk with death.
Finally, for the exploratory health care expenditure analysis, we used logistic regression to calculate the multivariable-adjusted odds ratios of an overall yearly expenditure> 85th percentile during 2016, comparing hyperkalemic and hypokalemic, respectively, to normokalemic patients (reference group), with adjustment for the potential confounders listed above.
Subgroup analysesAll analyses were performed overall, as well as for incident cases of each disease subgroup separately (CHF, CKD, DM, IHD, hypertension; not mutually exclusive). Additionally, the results were presented stratified by RAASi medication and by eGFR categories (defined based on median eGFR levels during the year preceding the index date).
Ethics in researchThe Ethics in Research Committee of our University Hospital and the Research Institute provided written approval to the study protocol.
ResultsStudy populationTable 1 displays the number of individuals included in each of the 4 analyses. The largest study population was that of the prevalent case analysis (n=36 269).
Baseline characteristics of the study participantsTable 2 summarizes the characteristics of the participants included in the prevalent case analysis. There were slightly more women (52.9%) than men, and the largest age stratum was 65 to 74 years. The most prevalent relevant condition was hypertension (90.5%) while IHD and CHF were the least frequent (8.2% and 7.9%, respectively). Most participants had a normal eGFR at baseline (≥ 60mL/min/1.73 m2). Similar trends were observed in the study population included in the prevalent case-prevalent user analysis. In contrast, the incident analyses included slightly more men than women, and the group aged 75 to 84 years was the largest age stratum. Once again, hypertension was the most frequent relevant condition (tables 7-9 of the supplementary data).
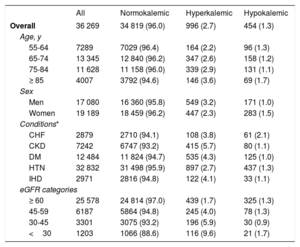
Baseline characteristics of the study participants included in the prevalent case analysis, overall and by K+ profile
| All | Normokalemic | Hyperkalemic | Hypokalemic | |
|---|---|---|---|---|
| Overall | 36 269 | 34 819 (96.0) | 996 (2.7) | 454 (1.3) |
| Age, y | ||||
| 55-64 | 7289 | 7029 (96.4) | 164 (2.2) | 96 (1.3) |
| 65-74 | 13 345 | 12 840 (96.2) | 347 (2.6) | 158 (1.2) |
| 75-84 | 11 628 | 11 158 (96.0) | 339 (2.9) | 131 (1.1) |
| ≥ 85 | 4007 | 3792 (94.6) | 146 (3.6) | 69 (1.7) |
| Sex | ||||
| Men | 17 080 | 16 360 (95.8) | 549 (3.2) | 171 (1.0) |
| Women | 19 189 | 18 459 (96.2) | 447 (2.3) | 283 (1.5) |
| Conditions* | ||||
| CHF | 2879 | 2710 (94.1) | 108 (3.8) | 61 (2.1) |
| CKD | 7242 | 6747 (93.2) | 415 (5.7) | 80 (1.1) |
| DM | 12 484 | 11 824 (94.7) | 535 (4.3) | 125 (1.0) |
| HTN | 32 832 | 31 498 (95.9) | 897 (2.7) | 437 (1.3) |
| IHD | 2971 | 2816 (94.8) | 122 (4.1) | 33 (1.1) |
| eGFR categories | ||||
| ≥ 60 | 25 578 | 24 814 (97.0) | 439 (1.7) | 325 (1.3) |
| 45-59 | 6187 | 5864 (94.8) | 245 (4.0) | 78 (1.3) |
| 30-45 | 3301 | 3075 (93.2) | 196 (5.9) | 30 (0.9) |
| <30 | 1203 | 1066 (88.6) | 116 (9.6) | 21 (1.7) |
CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; IHD, ischemic heart disease; K+, potassium.
Results are presented as No. (%); %s are by rows. Percentages may not add up to 100% due to rounding. All P values for chi-squared comparisons across K+ categories were <.001.
Individuals included in this analysis were those who had at least one relevant condition as of January 1st, 2016.
Among the study population included in the prevalent case analysis (table 2), the vast majority were normokalemic (96%) during the 3-month evaluation period, and there were more than twice the number of hyperkalemic (2.7%) than hypokalemic (1.3%) individuals. The prevalence of hyperkalemia was higher in men than in women, and increased with increasing age and with decreasing eGFR (9.6% among those with eGFR <30). Among relevant conditions, the highest prevalence of hyperkalemia was observed in individuals with CKD. Hypokalemia was more frequent among women and among patients with CHF.
Similar trends for hyper- and hypokalemia were observed among prevalent cases using at least 1 RAASI medication as of January 1st, 2016 (table 8 of the supplementary data), although the prevalence of hyperkalemia was slightly higher in this population, particularly in specific subgroups (eg, a prevalence of 11.0% among those with eGFR <30). The use of MRA was associated with a higher prevalence of hyperkalemia (4.8%) compared with the use of ACEIs and ARBs. In incident case and incident case-incident user analyses (tables 7-9 of the supplementary data), the overall frequency of hyperkalemia was higher (4.0% and 4.4%, respectively) than that observed in the prevalent case analyses. The same was true for hypokalemia (2.3% and 2.1%, respectively).
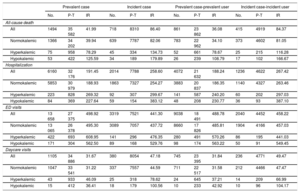
Crude incidence of study outcomes during follow-upTable 3 presents the crude incidence rates of the study outcomes during follow-up overall and by K+ profile. Emergency department visits were the most frequent event (incidence rates ranging from 441 to 498 per 1000 person-years) while all-cause death and hospital daycare visits were the least frequent. For all study outcomes, higher rates were observed in the incident compared with the prevalent analyses. In all analyses, hyperkalemia was associated with higher crude incidence rates of all-cause death compared with patients with normokalemia (incidence rates in the prevalent case analysis of 39.94 and 78.29 per 1000 person-years, respectively). However, the highest rates of all-cause death occurred among individuals with hypokalemia (incidence rate in the prevalent case analysis of 125.59 per 1000 person-years). Hyperkalemia and hypokalemia were also associated with higher crude incidence rates of hospitalization, emergency department visits, and hospital daycare visits compared with patients with normokalemia. More specifically, for hospitalization and emergency department visits, the crude incident rate for prevalent (case and user) analyses was higher for hyperkalemic patients than for the other groups. In contrast, in terms of incident analyses, these rates were higher for hypokalemic patients than for the other groups.
Incidence rates of the study outcomes during follow-up for prevalent and incidents analyses, overall and by K+ profile
| Prevalent case | Incident case | Prevalent case-prevalent user | Incident case-incident user | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | P-T | IR | No. | P-T | IR | No. | P-T | IR | No. | P-T | IR | |
| All-cause death | ||||||||||||
| All | 1494 | 35 582 | 41.99 | 718 | 8310 | 86.40 | 861 | 23 862 | 36.08 | 415 | 4919 | 84.37 |
| Normokalemic | 1366 | 34 202 | 39.94 | 639 | 7787 | 82.06 | 783 | 22 962 | 34.10 | 373 | 4602 | 81.05 |
| Hyperkalemic | 75 | 958 | 78.29 | 45 | 334 | 134.73 | 52 | 661 | 78.67 | 25 | 215 | 116.28 |
| Hypokalemic | 53 | 422 | 125.59 | 34 | 189 | 179.89 | 26 | 239 | 108.79 | 17 | 102 | 166.67 |
| Hospitalization | ||||||||||||
| All | 6160 | 32 176 | 191.45 | 2014 | 7788 | 258.60 | 4072 | 21 632 | 188.24 | 1236 | 4622 | 267.42 |
| Normokalemic | 5853 | 30 979 | 188.93 | 1863 | 7327 | 254.27 | 3883 | 20 837 | 186.35 | 1140 | 4327 | 263.46 |
| Hyperkalemic | 223 | 828 | 269.32 | 92 | 307 | 299.67 | 141 | 587 | 240.20 | 60 | 202 | 297.03 |
| Hypokalemic | 84 | 369 | 227.64 | 59 | 154 | 383.12 | 48 | 208 | 230.77 | 36 | 93 | 387.10 |
| ED visits | ||||||||||||
| All | 13 658 | 27 375 | 498.92 | 3319 | 7521 | 441.30 | 9038 | 18 491 | 488.78 | 2040 | 4452 | 458.22 |
| Normokalemic | 13 065 | 26 378 | 495.30 | 3089 | 7057 | 437.72 | 8660 | 17 826 | 485.81 | 1904 | 4166 | 457.03 |
| Hyperkalemic | 422 | 693 | 608.95 | 141 | 296 | 476.35 | 280 | 491 | 570.26 | 86 | 195 | 441.03 |
| Hypokalemic | 171 | 304 | 562.50 | 89 | 168 | 529.76 | 98 | 174 | 563.22 | 50 | 91 | 549.45 |
| Daycare visits | ||||||||||||
| All | 1105 | 34 886 | 31.67 | 380 | 8054 | 47.18 | 745 | 23 395 | 31.84 | 236 | 4771 | 49.47 |
| Normokalemic | 1047 | 33 541 | 31.22 | 337 | 7557 | 44.59 | 711 | 22 517 | 31.58 | 212 | 4466 | 47.47 |
| Hyperkalemic | 43 | 933 | 46.09 | 25 | 318 | 78.62 | 24 | 645 | 37.21 | 14 | 209 | 66.99 |
| Hypokalemic | 15 | 412 | 36.41 | 18 | 179 | 100.56 | 10 | 233 | 42.92 | 10 | 96 | 104.17 |
ED, emergency department; IR, incidence rate; K+, potassium; No., number; P-T, person-time.
Follow-up for prevalent analyses was up to 12 months, and incident analyses were up to 21 months.
Incidence rates are expressed per 1000 person-years.
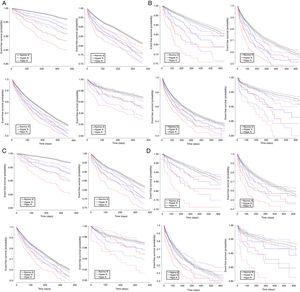
Consistent results were observed in analyses using Kaplan-Meier survivor function plots (figure 1A-D). There was a trend toward worse event-free survival in participants with hyperkalemia and with hypokalemia compared with normokalemia for the 4 clinical study outcomes. The 95% confidence intervals (95%CI) were narrower in the prevalent analyses and wider in the incident analyses. Finally, differences in event-free survival between individuals with K+ abnormalities and normokalemic participants were particularly salient for all-cause death, the worst prognosis being observed in participants with hypokalemia.
Kaplan-Meier cumulative survivor function curves for all-cause death (upper left), hospitalization (upper right), ED visits (lower left) and daycare visits (lower right), in the prevalent case analysis (A), incident case analysis (B), prevalent case-prevalent user analysis (C), and incident case-incident user analysis (D). ED, emergency department; Hyper K, hyperkalemia; Hypo K, hypokalemia; Normo K, normokalemia.
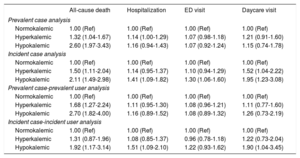
Table 4 summarizes the results of the multivariable-adjusted regression analyses comparing the risk of each of the 4 study outcomes among hyperkalemic and hypokalemic (compared with normokalemic) individuals, adjusting for potential confounders. Hyperkalemia was associated with an increased risk of all-cause death compared with normokalemia, ranging from a 31% to 68% increased risk depending on the analytic approach used. The 95%CI included the null value only in the incident case-incident user analysis. The risk of death was even higher among individuals with hypokalemia, the HR ranging from 1.92 to 2.70 depending on the analytic approach used, with all 95%CI above 1.00.
Associations between K+ abnormalities and clinical study outcomes during follow-up
| All-cause death | Hospitalization | ED visit | Daycare visit | |
|---|---|---|---|---|
| Prevalent case analysis | ||||
| Normokalemic | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Hyperkalemic | 1.32 (1.04-1.67) | 1.14 (1.00-1.29) | 1.07 (0.98-1.18) | 1.21 (0.91-1.60) |
| Hypokalemic | 2.60 (1.97-3.43) | 1.16 (0.94-1.43) | 1.07 (0.92-1.24) | 1.15 (0.74-1.78) |
| Incident case analysis | ||||
| Normokalemic | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Hyperkalemic | 1.50 (1.11-2.04) | 1.14 (0.95-1.37) | 1.10 (0.94-1.29) | 1.52 (1.04-2.22) |
| Hypokalemic | 2.11 (1.49-2.98) | 1.41 (1.09-1.82) | 1.30 (1.06-1.60) | 1.95 (1.23-3.08) |
| Prevalent case-prevalent user analysis | ||||
| Normokalemic | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Hyperkalemic | 1.68 (1.27-2.24) | 1.11 (0.95-1.30) | 1.08 (0.96-1.21) | 1.11 (0.77-1.60) |
| Hypokalemic | 2.70 (1.82-4.00) | 1.16 (0.89-1.52) | 1.08 (0.89-1.32) | 1.26 (0.73-2.19) |
| Incident case-incident user analysis | ||||
| Normokalemic | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Hyperkalemic | 1.31 (0.87-1.96) | 1.08 (0.85-1.37) | 0.96 (0.78-1.18) | 1.22 (0.73-2.04) |
| Hypokalemic | 1.92 (1.17-3.14) | 1.51 (1.09-2.10) | 1.22 (0.93-1.62) | 1.90 (1.04-3.45) |
ED, emergency department; K+, potassium.
The analyses for hospitalization, ED visits and daycare visits accounted for the competing risk with death. Models were adjusted for age, sex, individual income, comorbidities, estimated glomerular filtration rate, and medication use (all analyses); plus disease duration, prior hospitalizations, prior ED visits, and prior serum K+ measurements (prevalent case and prevalent case-prevalent user analyses).
With regards to the other 3 clinical outcomes, weaker trends toward increased risks of hospitalization, emergency department visits, and hospital daycare visits were observed in individuals with hyperkalemia compared with those with normokalemia, although all 95%CIs included the null value. For hypokalemia, in incident case analyses there were strong, statistically significant associations with hospitalization (HR, 1.41; 95%CI, 1.09-1.82), emergency department visits (HR, 1.30; 95%CI, 1.06-1.60) and hospital daycare visits (HR, 1.95; 95%CI, 1.23-3.08). The incident case-incident user analysis yielded similar findings in patients with hypokalemia, although 95%CIs were wider.
Subgroup analysesSubgroup analyses by prevalent/incident condition were limited by the small absolute number of events occurring in specific strata (tables 10-12 of the supplementary data), and could not be conducted in the incident case-incident user population. In the prevalent case analysis, the strongest associations between K+ derangements and all-cause death were observed in CKD patients.
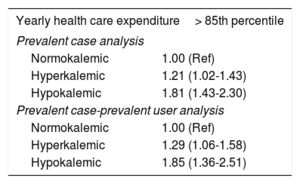
Associations between K+ derangements and yearly health care expenditureIn the exploratory analyses assessing yearly health care expenditure among prevalent cases (table 5), patients with hyperkalemia had higher multivariable-adjusted odds of having a yearly health care expenditure> 85th percentile compared with normokalemic patients, the increased odds ranging from 21% to 29%. This association was even stronger for hypokalemic than for normokalemic patients (increased odds ranging from 81% to 85%).
Associations between K+ abnormalities and yearly health care expenditure
| Yearly health care expenditure> 85th percentile | |
|---|---|
| Prevalent case analysis | |
| Normokalemic | 1.00 (Ref) |
| Hyperkalemic | 1.21 (1.02-1.43) |
| Hypokalemic | 1.81 (1.43-2.30) |
| Prevalent case-prevalent user analysis | |
| Normokalemic | 1.00 (Ref) |
| Hyperkalemic | 1.29 (1.06-1.58) |
| Hypokalemic | 1.85 (1.36-2.51) |
K+, potassium.
Models were adjusted for age, sex, individual income, comorbidities, estimated glomerular filtration rate, medication use, disease duration prior hospitalizations, prior emergency department visits, and prior serum K+ measurements.
We conducted a population-based, longitudinal analysis including a large population of patients with either prevalent or incident chronic cardio-metabolic conditions. Hyperkalemia was twice as frequent as hypokalemia among patients with deranged serum potassium levels, and was more frequent among men and older participants, while hypokalemia was more frequent among women. Regardless of the analytic approach used, compared with normokalemic patients, those with hyperkalemia had a worse crude event-free survival for all-cause death, hospitalization, emergency department visits, and daycare visits. Crude event-free survival was even worse for hypokalemic patients. On multivariable-adjusted analyses, hyperkalemia was robustly and significantly associated with an increased risk of all-cause death (ranging from a 31% to 68% increased risk) and with an increased odds of a yearly health care expenditure> 85th percentile (ranging from 21% to 29% increased odds). These associations were even stronger in hypokalemic patients.
To our knowledge, this is the first study to evaluate the real-world importance of K+ derangements (including both hyper- and hypokalemia, identified using laboratory test data from both hospital and primary care settings) and their associations with key clinical outcomes, including all-cause death, health care resource use, and health-related expenditure. Prior studies have mostly focused on hyperkalemia alone, and/or on a smaller set of chronic underlying conditions.14 This, together with some inconsistent findings reported in some studies, emphasized the need for further research in this field, assessing both clinically relevant K+ abnormalities (hyper- and hypokalemia) and across multiple chronic conditions.
Our findings specifically for hyperkalemia and its association with all-cause death and hospitalization are consistent with those from prior studies, which were restricted to hyperkalemia as the exposure of interest.15–17 For example, in a Danish cohort of CHF patients, hyperkalemia was associated with several adverse outcomes including a higher multivariable-adjusted risk of all-cause death. Of note, in that study, which used a longer evaluation period for serum K+ levels than ours, almost 40% of CHF patients developed hyperkalemia during follow-up, and recurrent hyperkalemia was also a frequent phenomenon.16 In an analysis using data from the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS), both from the US, hyperkalemia was also associated with all-cause and cardiovascular death in apparently healthy individuals from the general population.17 These studies did not assess hypokalemia, other chronic conditions, or health care expenditure.
Our results are also consistent with those from the few analyses in which the full spectrum of K+ levels was assessed in patients with specific chronic conditions. Most of them have described a U-shaped relationship between serum K+ levels and the risk of key clinical outcomes, including hospitalization and death. For instance, in a clinical cohort of patients with CHF followed up in the outpatient setting, Núñez et al.18 observed that serum K+ levels were independently associated with mortality. Similar to our results, the authors observed that hypokalemic patients had an even higher risk of death than those with hyperkalemia when these groups were compared with normokalemic patients. Conversely, Collins et al., 5 observed a J-shaped relationship between K+ levels and clinical outcomes in patients with CKD, DM and CHF, with slightly stronger associations for hyperkalemia than for hypokalemia. Compared with our study, hypertension and IHD were not included, and the authors adjusted for a more limited set of potential confounders (eg, no measure of disease severity was included, and analyses were not adjusted for socioeconomic features). Furthermore, no health expenditure analyses were conducted in any of these studies.
Our study has important clinical implications. The strong, independent associations between K+ derangements and hard clinical outcomes observed in our multivariable-adjusted analyses provide a further, observational rationale to maintaining normokalemia in these patients, and suggest that actions aimed at preventing serum K+ abnormalities in patients with chronic conditions at high risk of hyper- and hypokalemia (eg, patients with CHF, particularly those using RAASI medications and multiple diuretics) could potentially reduce mortality, hospital admissions, and health care costs. The fact that the associations were consistent regardless of which of the 4 analytic approaches was used further reinforces our conclusions. Therefore, we can hypothesize that K+ binders aimed at reducing the risk of hyperkalemia, which stabilize K+ homeostasis in patients with these chronic conditions, will potentially play a relevant role in the management these patients, particularly among those treated with RAASI medications, which are class I medications in the management of conditions such as CHF but are known to increase the risk of hyperkalemia. Similarly, the use of potassium supplements to treat or prevent hypokalemia in patients with CHF in whom diuretic therapy is up-titrated may also have important implications for the future standard management of these patients. Nevertheless, experimental studies are needed to confirm these observations and hypotheses.
Study strengthsOur study has important strengths compared with prior research in this field. We used a very large population database and included a large number of patients with each of the relevant conditions. This increased overall statistical power and precision compared with previous studies conducted in smaller clinical cohorts. Modeling a time-varying, potentially recurrent exposure such as serum K+ levels is challenging, and a number of modeling approaches have been described in the literature. The design becomes even more complex when considering exposure to specific drugs, and pharmacoepidemiological methods are necessary. Therefore, for the present analysis, we used a variety of study designs and analytic approaches, aimed at maximizing the robustness of the results and at minimizing their sensitivity to specific study design assumptions and potential biases. The consistency of the observed results across analytic approaches further reinforces the validity of our findings.
In addition, to minimize the possibility of residual confounding, adjustments for a number of potential confounders were conducted. Specifically, detailed, updated data on baseline eGFR was used to ensure adequate adjustment for this key covariate. Because eGFR and K+ levels are strongly correlated, this information was obtained before (rather than during) the evaluation period for each participant, which allowed adjustment for baseline eGFR while minimizing collinearity and over-adjustment.
Study limitationsThe present study also has some limitations. First, as for any analysis using information generated from routine care and recorded in large administrative health care databases, under-recording of health conditions is possible. This may have led to some underestimation of the relevant cardio-metabolic conditions assessed, but this is unlikely to have affected the reported associations among individuals with recorded conditions. In addition, some residual confounding on adjustment for comorbidities in the multivariable analyses cannot be ruled out. Nevertheless, the multivariable analytic strategy used was very comprehensive, including adjustment for adjusted morbidity groups, a summary measure of complexity and comorbidity, and for other clinically relevant features such as disease duration and prior hospitalizations, which are expected to be able to capture baseline differences in the likelihood of incident events across groups.
Second, although the overall sample size of the study was very large, the number of patients in specific subgroups was relatively small. This limited our ability to evaluate the associations in some specific subgroups such as those patients with recurrent hyper- or hypokalemia and those with mixed K+ derangements. Of note, inclusion in the study population of individuals with no K+ measurements—who were excluded from the analysis—would not have ameliorated this, as the small counts issue affected hyper- and hypokalemic strata rather than normokalemic groups. Since small numbers of events are a frequent issue in pharmacoepidemiological research studies, larger studies combining multiple population-based databases may be necessary to characterize the associations between K+ derangements and incident events in specific subgroups of patients.
Finally, our health care expenditure analyses used a published calculation for overall health care expenditure. Nonetheless, this calculation is intended to be used for a given whole calendar year (eg, 2016) rather than for time periods of varying lengths, such as those allowed for in our incident analyses. Consequently, such analyses could not be performed in the incident designs. More generally, due to their limited granularity, these analyses should be considered hypothesis-generating, and more detailed health economics evaluations should be considered in smaller clinical cohorts.
ConclusionsIn this large, population-based, longitudinal study including 36 269 patients with either prevalent or incident chronic cardiovascular-metabolic-renal conditions (CHF, CKD, DM, hypertension, or IHD), hyperkalemia was robustly and independently associated with an increased risk of all-cause death and with increased health care expenditure, compared with patients with normokalemia. These associations were even stronger in hypokalemic patients. Although experimental studies are needed to better understand the safety and efficacy of interventions aimed at stabilizing K+ homeostasis in patients with chronic cardio-metabolic conditions, for now, our findings provide robust observational evidence of the importance of maintaining normal K+ levels in such patients who are often at high risk of developing K+ derangements.
FundingThe present study was funded by an unrestricted research grant from Vifor Pharma.
Conflicts of InterestJ. Comín-Colet has received speaker fees from Vifor Pharma. J. Comín-Colet and M. Cainzos-Achirica have participated in other research projects funded by unrestricted grants from Vifor Pharma. The rest of authors have no conflicts of interest.
- -
Potassium derangements have been previously studied, especially hyperkalemia, across multiple chronic conditions separately, particularly CKD.
- -
Hyperkalemia seems to be associated with worse clinical outcomes, at least in CHF with left ventricular ejection fraction <40% and CKD. Hypokalemia has been analyzed less frequently.
- -
Prior studies have reported some inconsistent findings, emphasizing the need for further research in this field.
- -
Our results are consistent with those of prior studies that related hyperkalemia and clinical outcomes and with those from the few analyses assessing the full spectrum of K+ levels.
- -
This study adds a population-based overview of potassium derangements across a complete spectrum of 5 chronic cardiovascular conditions, common in daily clinical practice (CKD, CHF, DM, hypertension, IHD).
- -
We evaluated not only the frequency of hyper- and hypokalemia, but also their clinical association with key outcomes such as mortality and hospitalization.
- -
In prior analyses, the chronic conditions were evaluated separately in different populations, which were less comparable.
- -
To our knowledge, this is the first study to evaluate the association between potassium derangements, clinical outcomes, and health costs and expenditure.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.06.013