Thrombus aspiration allows analysis of intracoronary material in patients with ST–segment elevation myocardial infarction. Our objective was to characterize this material by immunohistology and to study its possible association with patient progress.
MethodsThis study analyzed a prospective cohort of 142 patients undergoing primary angioplasty with positive coronary aspiration. Histological examination of aspirated samples included immunohistochemistry stains for the detection of plaque fragments. The statistical analysis comprised histological variables (thrombus age, degree of inflammation, presence of plaque), the patients’ clinical and angiographic features, estimation of survival curves, and logistic regression analysis.
ResultsAmong the histological markers, only the presence of plaque (63% of samples) was associated with postinfarction clinical events. Factors associated with 5-year event–free survival were the presence of plaque in the aspirate (82.2% vs 66.0%; P = .033), smoking (82.5% smokers vs 66.7% nonsmokers; P = .036), culprit coronary artery (83.3% circumflex or right coronary artery vs 68.5% anterior descending artery; P = .042), final angiographic flow (80.8% II-III vs 30.0% 0–I; P < .001) and left ventricular ejection fraction ≥ 35% at discharge (83.7% vs 26.7%; P < .001). On multivariable Cox regression analysis with these variables, independent predictors of event-free survival were the presence of plaque (hazard ratio, 0.37; 95%CI, 0.18-0.77; P = .008), and left ventricular ejection fraction (hazard ratio, 0.92; 95%CI, 0.88-0.95; P < .001).
ConclusionsThe presence of plaque in the coronary aspirate of patients with ST elevation myocardial infarction may be an independent prognostic marker. CD68 immunohistochemical stain is a good method for plaque detection.
Keywords
The genesis of arterial thromboses involves 3 factors (Virchow's triad): arterial wall characteristics, blood flow properties, and certain circulating factors in the blood. In autopsy studies of individuals with sudden cardiac death, thrombosis associated with plaque rupture appears to be the predominant mechanism underlying the occurrence of acute coronary syndrome. This pathogenic process can begin days or even weeks before a fatal ST-segment elevation myocardial infarction (STEMI),1,2 and intraplaque hemorrhage, caused by cracks or fissures in the surface of the arterial lumen, can accelerate the transition toward rupture.3
Many ruptured atheroma plaques are initially covered by wall thrombi and have no clinical symptoms.4 Such wall thrombi can become organized over time and incorporated into atherosclerotic lesions, restoring the integrity of the vessel wall. This type of “scar” lesion is often seen in coronary arteries in autopsies.4–6 In contrast, in other patients, atherosclerotic plaque rupture triggers a process of repeated or continuous thrombosis that culminates in acute coronary syndrome.7,8
The use of thrombus aspiration during primary percutaneous coronary intervention (PCI) offers a unique opportunity to study the composition of thrombi and their dynamic in vivo formation.9 Although the samples obtained using thrombus aspiration fail to completely reflect all components of thrombi or their actual distribution, they allow thrombi to be studied without the effect of postmortem histological changes.
Various researchers have studied the histological characteristics of aspirated samples in patients with STEMI, analyzing the composition of the samples and the age of the coronary thrombus. In a large proportion of patients, the thrombus age is older than 24hours, confirming that sudden coronary occlusion is often preceded by a variable period of plaque instability and thrombus formation.10,11
Only 1 study12 has analyzed the relationship between coronary aspirate histology and the prognosis of STEMI patients, finding that thrombus age was an independent predictor of long-term mortality.
The aim of the present study was to immunohistologically characterize the coronary material aspirated during primary PCI and analyze its ability to predict clinical events in patients with STEMI.
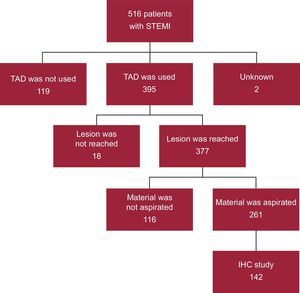
METHODSStudy ParticipantsThe study cohort comprised 142 patients with STEMI (Figure 1). Aspirate material had been extracted from the patients during primary PCI using a coronary thrombus aspirator. Samples were collected between January 2005 and August 2007 and throughout 2009 (in 2008, the hospital moved to its current location, precluding the analysis of aspirated thrombi). Samples were collected consecutively on weekdays from 0800 to 1500hours for logistical reasons (absence of personnel for sample shipment and collection). Thus, although aspirate material was collected from 261 primary PCIs during the study period, this study included 142 samples.
Clinical, angiographic, and procedure-related variables were prospectively collected in a database specifically designed for this purpose. The information recorded included epidemiological characteristics and patients’ medical records, presence of prodromal angina, Killip class, ST resolution, analytical parameters, left ventricular ejection fraction (LVEF) at discharge, procedure times (eg, symptom onset-to-balloon, door-to-balloon), angioplasty information, and patient outcomes.
All angiograms were reviewed, with various details recorded, such as the presence of collateral circulation (0-3, according to the Rentrop classification13), TIMI (Thrombolysis in Myocardial Infarction) flow prior to PCI, and the final angiographic result. This result was evaluated using the final TIMI flow, the corrected TIMI Frame Count,14 and the degree of myocardial perfusion, classified from 0 to 3 according to the definition of the TIMI Group.15
Drug therapy prior to PCI included aspirin and clopidogrel in loading doses of 300 and 600mg, respectively, and 5000 to 10 000 IU of unfractionated heparin. Glycoprotein IIb/IIIa inhibitors were used systematically, unless contraindicated, and the aspiration device was chosen by the operator for each procedure.
Processing and Analysis of Intracoronary MaterialThe aspirated samples were washed with saline immediately after extraction, fixed in 10% neutral buffered formalin, and sent to the department of anatomic pathology for processing. After fixation, the material was embedded in paraffin, sectioned to obtain 4-¿m slices, and mounted on glass slides. The sections were stained with hematoxylin and eosin for light microscopy and with antibodies (Dako and Glostrup, Denmark) against macrophages (CD68), platelet-endothelial cell adhesion molecule (CD31), and actin filaments (HHF35). Perls staining to detect iron could be performed in 111 samples to identify areas of old bleeding in the interior of the plaque fragments (Figure 2).
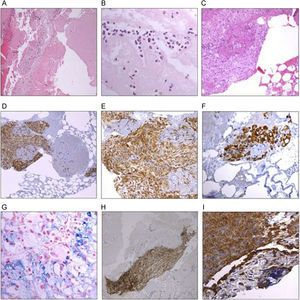
Photomicrographs of coronary material extracted via thrombus aspiration from patients with ST-segment elevation myocardial infarction. A: heterogeneous thrombus with areas of fresh thrombi that include intact blood cells (granulocytes, platelets, and erythrocytes) and fibrin, together with lytic areas with cellular degradation. B: detail of a fresh thrombus showing eosinophils and neutrophils with fibrin networks. C: coronary aspirate material that includes atheroma fragments composed of macrophages. D, E, and F: representative images of atheroma plaque with immunohistochemical stains: cells positive for CD68 (macrophages) permit identification of “soft plaque” fragments. G: detail of Perls staining for iron, with the siderophages permitting the identification of areas of old bleeding. H: atheroma plaque fragments identified using positive staining for HF35 (actin) antibodies. I: cells stained with antibodies against platelet-endothelial cell adhesion molecule (CD31) indicative of an organized thrombus.
An experienced pathologist blinded to patient information performed the histopathological analysis. According to the previously accepted histopathological definition,10 the thrombus age was classified into 2 groups: fresh thrombi (< 1 day), composed of layers of fibrin, platelets, erythrocytes, and intact granulocytes, and old thrombi (> 1 day), composed of lytic (1-5 days) and/or organized (> 5 days) areas. Lytic areas are characterized by colliquation necrosis and granulocyte karyorrhexis, whereas organized areas are characterized by the development of new capillary vessels and smooth muscle cells, with or without connective tissue deposits. In the present analysis, we distinguished fresh and old thrombi, with old thrombi containing at least 1 lytic or organized area.
The Perls staining and immunohistochemistry were semiquantitatively analyzed and graded as follows: 0, negative staining; 1, few positive cells; and 2, many positive cells. For the statistical analysis, samples scoring 1 or 2 were grouped and those scoring 0 were considered negative.
Plaque fragments were identified by the presence of some constituent material of “soft plaque” (extracellular lipids, macrophage foam cells [CD68 positive], and/or cholesterol crystals), smooth muscle fibers (HHF35), endothelial cells (CD31), iron deposits, calcium, fibroelastic tissue, or their combinations. Staining with CD68 and CD31 was only considered positive when it marked plaque components: macrophages in the case of CD68—present in the plaque and not in the thrombus—and endothelial cells with CD31.
The local ethics committee approved the handling and analysis of the aspirated samples and all patients agreed to participate in the study. The procedures used were carried out in accordance with the guidelines of the Declaration of Helsinki concerning the ethical principles for medical research in humans.
Clinical Follow-upPatient follow-up was performed via an outpatient clinic or telephone contact for a median of 2.4 years (1-6 years), with a loss to follow-up of 1%. Three physicians (A. Blasco, L. Goicolea, and A. Muñiz) in charge of the database prospectively recorded major adverse cardiac events (MACE): death, recurrent myocardial infarction, urgent revascularization, and definite or probable stent thrombosis (Academic Research Consortium).16
Statistical AnalysisCategorical variables are expressed as percentages and continuous variables as mean ± standard deviation or as median [interquartile range] for times. Associations between qualitative variables were analyzed using the chi-square test and those between continuous variables using the Student t test and Mann-Whitney U test. Survival curves for MACE were estimated using the Kaplan-Meier method and were compared using a log-rank test.
To identify independent predictors of MACE during follow-up, multivariable Cox regression analysis with backward selection was used, eliminating all variables scoring P < .05. The descriptive power of the model was evaluated using Harrell's C index,17 equivalent to the area under the ROC curve in survival models; C = .5 is no better than chance and C = 1 corresponds to perfect discrimination.
All tests were 2-sided and P < .05 was considered statistically significant. Statistical analysis was performed with SPSS version 18. 0.
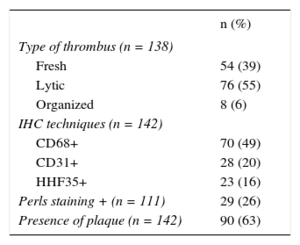
RESULTSThe study included 142 patients with STEMI whose material was obtained via aspiration during primary PCI. The histological characteristics of the coronary aspirates are shown in Table 1.
Histological and Immunohistochemical Characteristics of Coronary Aspirates in Patients With STEMI
| n (%) | |
|---|---|
| Type of thrombus (n = 138) | |
| Fresh | 54 (39) |
| Lytic | 76 (55) |
| Organized | 8 (6) |
| IHC techniques (n = 142) | |
| CD68+ | 70 (49) |
| CD31+ | 28 (20) |
| HHF35+ | 23 (16) |
| Perls staining + (n = 111) | 29 (26) |
| Presence of plaque (n = 142) | 90 (63) |
CD31, antibody against platelet-endothelial cell adhesion molecule; CD68, macrophage marker; HHF35, antibodies against actin filaments; IHC, immunohistochemistry; STEMI, ST-segment elevation myocardial infarction.
In 4 of the 142 samples studied, no thrombus was identified or it was insufficient to permit categorization of its age. In the remaining 138 samples, the thrombus was fresh in 54 (39%), lytic in 76 (55%), and organized in 8 (6%). Granulocyte infiltration was absent or mild in 77 of the 138 patients (56%) and moderate or intense in 61 (44%).
Atherosclerotic plaque components were identified in 90 of the 142 patients (63%). Antibody staining of macrophages (CD68) was positive in 49% of the samples; only 6 samples negative for CD68 were positive in either of the 2 immunohistochemical techniques (HHF35 in 5 samples, CD31 in 2). In 4 samples, the plaque was identified via hemosiderin deposits (Perls+).
The median weight of the aspirated samples was 0.046g [0.02-0.1g]. Univariable analysis showed that aspirate weight was inversely associated with the presence of plaque (median weight without plaque, 0.07g [0.03-0.12g]; with plaque, 0.04g [0.02-0.10g]; P = .017) Thus, the largest aspirates were less likely to contain plaque fragments.
The clinical and angiographic characteristics of the patients are summarized in Table 2. Clinical events occurring during follow-up were death from any cause in 24 patients (4.4/1000 person-months), cardiac death in 15 (2.7/1000 person-months), and reinfarction in 7 (1.3/1000 patient-months). The composite MACE outcome occurred in 32 patients (7/1000 patient-months).
Clinical and Angiographic Characteristics of Patients and Association of Variables Prior to Coronary Aspiration With the Presence of Plaque in the Aspirate
| Total | Without plaque | Plaque | P | |
|---|---|---|---|---|
| Prior to coronary aspiration | ||||
| Male sex | 116 (81.7) | 36 (32.4) | 75 (67.6) | .340 |
| Age, y | 60 ± 13 | 60 ± 11 | 60 ± 14 | .917 |
| Smokers | 97 (68.3) | 31 (32.9) | 63 (67.0) | .628 |
| Hypertension | 68 (47.9) | 26 (40.6) | 38 (59.4) | .145 |
| Hypercholesterolemia | 54 (38.0) | 22 (51.5) | 31 (58.5) | .158 |
| Diabetes mellitus | 32 (22.5) | 10 (32.3) | 21 (67.7) | .785 |
| Preinfarction angina | 38 (27.7) | 14 (38.9) | 22 (61.1) | .407 |
| Multivessel disease | 43 (30.3) | 14 (33.3) | 28 (66.7) | .873 |
| Culprit AD artery | 54 (39.1) | 18 (35.3) | 33 (64.7) | .586 |
| Pre-PCI TIMI flow 0-I | 118 (88.7) | 41 (35.7) | 74 (64.3) | .134 |
| Collateral circulation | 45 (36.6) | 14 (31.8) | 30 (68.2) | .859 |
| Ischemia time, min* | 221 [170-310] | 217 [180-325] | 233 [170-310] | .620 |
| GPIIb/IIIa inhibitors | 112 (78.7) | 39 (84.8) | 68 (75.6) | .214 |
| After coronary aspiration | |
| Use of stent | 134 (95.0) |
| Stent diameter, mm | 3.3 ± 0.5 |
| ST decrease ≥ 50% after PCI | 87 (74.4) |
| Post-PCI TIMI flow II-III | 130 (92.9) |
| Post-PCI cTFC | 26 [21-41] |
| LVEF at discharge (%) | 47.1 ± 11.3 |
AD, anterior descending; cTFC, corrected TIMI frame count; GPIIb/IIIa, glycoprotein IIb/IIIa; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarct; TIMI, Thrombolysis in Myocardial Infarction.
Continuous variables are presented as means ± standard deviation or as the median [interquartile range] and categorical variables as n (%).
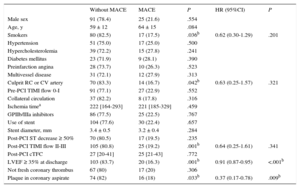
Table 3 shows a comparison of the clinical and angiographic characteristics of patients and the histology of the coronary aspirate according to the occurrence of adverse clinical events 5 years after STEMI. The presence of plaque in the aspirate was the only histological variable significantly associated with the occurrence of MACE, and no association was found with any other variable prior to aspiration that could be indicative of a confounding effect (Table 2). Thrombus age was not associated with patient outcomes in this series.
Comparison of Clinical and Angiographic Characteristics of Patients With and Without Adverse Events at 5 Years After STEMI (HRs Adjusted by Occurrence of MACE During Follow-up)
| Without MACE | MACE | P | HR (95%CI) | P | |
|---|---|---|---|---|---|
| Male sex | 91 (78.4) | 25 (21.6) | .554 | ||
| Age, y | 59 ± 12 | 64 ± 15 | .084 | ||
| Smokers | 80 (82.5) | 17 (17.5) | .036b | 0.62 (0.30-1.29) | .201 |
| Hypertension | 51 (75.0) | 17 (25.0) | .500 | ||
| Hypercholesterolemia | 39 (72.2) | 15 (27.8) | .241 | ||
| Diabetes mellitus | 23 (71.9) | 9 (28.1) | .390 | ||
| Preinfarction angina | 28 (73.7) | 10 (26.3) | .523 | ||
| Multivessel disease | 31 (72.1) | 12 (27.9) | .313 | ||
| Culprit RC or CV artery | 70 (83.3) | 14 (16.7) | .042b | 0.63 (0.25-1.57) | .321 |
| Pre-PCI TIMI flow 0-I | 91 (77.1) | 27 (22.9) | .552 | ||
| Collateral circulation | 37 (82.2) | 8 (17.8) | .316 | ||
| Ischemia timea | 222 [164-293] | 221 [185-329] | .459 | ||
| GPIIb/IIIa inhibitors | 86 (77.5) | 25 (22.5) | .767 | ||
| Use of stent | 104 (77.6) | 30 (22.4) | .657 | ||
| Stent diameter, mm | 3.4 ± 0.5 | 3.2 ± 0.4 | .284 | ||
| Post-PCI ST decrease ≥ 50% | 70 (80.5) | 17 (19.5) | .235 | ||
| Post-PCI TIMI flow II-III | 105 (80.8) | 25 (19.2) | .001b | 0.64 (0.25-1.61) | .341 |
| Post-PCI cTFC | 27 [20-41] | 25 [21-43] | .772 | ||
| LVEF ≥ 35% at discharge | 103 (83.7) | 20 (16.3) | .001b | 0.91 (0.87-0.95) | <.001b |
| Not fresh coronary thrombus | 67 (80) | 17 (20) | .306 | ||
| Plaque in coronary aspirate | 74 (82) | 16 (18) | .033b | 0.37 (0.17-0.78) | .009b |
95%CI, 95% confidence interval; cTFC, corrected TIMI frame count; CX, circumflex; GPIIb/IIIa, glycoprotein IIb/IIIa; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events (death, recurrent infarction, urgent revascularization, and/or stent thrombosis); PCI, percutaneous coronary intervention; RC, right coronary; STEMI, ST-segment elevation myocardial infarct; TIMI, Thrombolysis in Myocardial Infarction.
Continuous variables are presented as means ± standard deviation or as the median [interquartile range] and categorical variables as n (%).
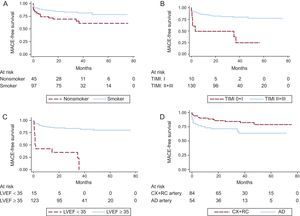
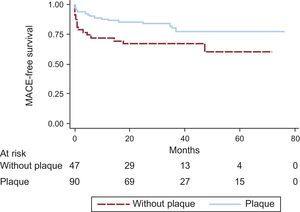
The survival curves for MACE were significantly different according to smoking habit, culprit coronary artery, TIMI flow after primary PCI, LVEF at discharge, and presence of plaque in the coronary aspirate (Figure 3 and Figure 4). The 5-year MACE-free survival rates were 82.5% in smokers vs 66.7% in nonsmokers (P = .036), 83.3% in patients with infarction of the circumflex or right coronary arteries vs 68.5% in those with infarction of the anterior descending artery (P = .042), 80.0% in patients with TIMI flow II-III vs 30.0% in those with a final TIMI flow 0-I (P < .001), 83.7% in patients with LVEF ≥ 35% vs 26.7% in those with LVEF < 35% before hospital discharge (P < .001), and 82.2% in patients whose samples contained plaque vs 66.0% in those with plaque-free aspirates (P = .033).
Comparative Kaplan-Meier curves of MACE-free survival rates. A: smokers vs nonsmokers. B: patients with TIMI flow II-III after percutaneous coronary intervention vs patients with final TIMI flow 0-I. C: patients with LVEF ≥ 35% vs patients with LVEF < 35% at discharge. D: patients with infarctions of the CX and RC arteries vs patients with infarctions of the AD artery. AD, anterior descending; CX, circumflex; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; RC, right coronary; TIMI, Thrombolysis in Myocardial Infarction.
The variables associated with the occurrence of adverse events in the univariable analysis were tested in the multivariable Cox regression model; independent predictors of long-term MACE were the presence of plaque fragments in the aspirate (hazard ratio [HR], 0.37; 95% confidence interval [95%CI], 0.18-0.77; P = .008) and LVEF (HR = 0.92; 95%CI, 0.88-0.95; P < .001) (Table 3). Harrell's C index of the model including the above 5 significant variables (plaque in the coronary aspirate, smoking habit, TIMI flow after PCI, LVEF at discharge, and culprit coronary artery) was .723, indicating a good predictive capacity for MACE. The variable “plaque in the coronary aspirate” improved the discriminative capacity of the model containing only 4 variables (C = .708).
DISCUSSIONThe main finding of this study is the association between the presence of atheroma in coronary aspirates from patients with STEMI and their event-free survival, an association that has, as far as we know, not been published previously. The reported rate of plaque fragments in the coronary aspirate ranges from 39% to 57%.10,18–20 In our series, the proportion was slightly higher (63%), possibly due to the use of immunohistochemical techniques facilitating their identification.
Only a single small series of 49 patients19 has studied the association between the presence of atheroma in the coronary aspirate and prognosis-related factors. This series analyzed the degree of opacification or myocardial blush, the analytically estimated size of the infarct (peak creatine kinase and its MB isoenyzme), and the LVEF. In the group of patients with plaque in the aspirate (57.1%), there was a greater proportion of myocardial blush grade 3 (82.1% vs 52.4%; P = .025), lower creatine kinase concentration (1705 ± 1647 vs 2629 ± 2013 U/L; P = .042) and higher LVEF (0.59 ± 0.10 vs 0.52 ± 0.08; P = .012). These findings coincide with ours, given that the presence of atheroma was associated with favorable prognostic markers.
In our series, 5 variables were associated with the long-term occurrence of MACE: LVEF at discharge, TIMI flow after PCI, culprit coronary artery, smoking, and the presence of atheroma plaque in the coronary aspirate. The influence of the first 3 variables, as well as the protective effect of smoking, concurs with the findings of other authors in large series of patients with acute myocardial infarction,21–23 although the cause of the last association is unclear. These 5 variables were included in the multivariable Cox regression model, and LVEF and presence of plaque in the aspirate were independent predictors of long-term MACE. Plaque aspiration was inversely associated with the cumulative rate of MACE.
On the basis of these results, we postulate that the presence of atheroma plaque in the coronary aspirates is associated with a better prognosis in patients with STEMI. The cause of this association cannot be clarified with our data. We can only speculate that the aspirates containing atheroma fragments could identify a group of patients with larger and/or more unstable plaques, in contrast to the other group with more thrombotic component but with less atheroma. This finding could be reflected in the 2 different mechanisms underlying coronary occlusion: the first, mainly due to the substrate of the arterial wall (plaque rupture), and the second, dependent above all on systemic factors in circulating blood. However, the collection of plaque could also be the consequence of more complete or successful aspiration, although in our series the aspirate weight was inversely associated with the presence of atheroma fragments, making this last theory unlikely.
In most patients, plaque fragments were identified using differential staining of macrophages with a specific antibody (CD68). Staining with CD68 was the most useful technique for this purpose, although in some patients (17%), the plaque had no macrophages and was identified via the presence of smooth muscle fibers, endothelial cells, cholesterol crystals, or iron deposits.
The proportion of macrophages present in the plaque after carotid endarterectomy can also predict restenosis. Helling et al.24 found that patients with plaques rich in macrophages had a higher risk of restenosis (odds ratio [OR] = 0.43; 95%CI, 0.26-0.72), independently of other factors. In additional, the existence of a large lipid core size (> 40%) was associated with lower risk of restenosis (OR = 0.40; 95%CI, 0.19-0.81). Our data did not allow us to explore this hypothesis, given that follow-up examinations did not include angiography. This aspect could represent a future research target.
Various authors have histologically analyzed coronary aspirates from patients with STEMI in series of various sizes, focusing on different histopathological aspects.11,12,18,19,25–28 The largest series was published by Kramer et al.,12 with 1315 patients. In that study, 40% of coronary aspirates contained lytic or organized thrombus (> 1 day), and this histological characteristic was independently associated with long-term mortality (HR = 1.83; 95CI%, 1.14-2.93; P = .01). However, the authors recognized that the risk profile of patients with older thrombus could differ from that of patients with fresh thrombus. The presence of plaque was not associated with survival but they performed no immunohistochemical analysis. In our patients, this type of analysis was vital for aspirate characterization.
In another study of 892 patients,11 the risk of incomplete ST-segment recovery tended to be higher in patients with older thrombi (OR = 1.33; 95CI%, 0.95-1.85; P = .097) and the ST recovery was a strong predictor of long-term mortality. Although our data fail to support the relationship between thrombus age and clinical outcomes, there was an association between thrombus age and ischemia time: total ischemia time (symptom onset-to-balloon) in patients with older thrombus was almost double that of patients with fresh thrombus (4.3 and 2.4hours, respectively; P = .007).
LimitationsOur study has several limitations. First, the small number of patients increases the possibility of associations among variables being missed. This shortcoming also stops us from independently analyzing the components of the composite outcome MACE. Second, the formation of arterial thrombi depends not only on the thrombogenicity of the vascular wall, but also circulating factors and blood flow characteristics. No evaluation was performed of these factors. Third, the aspiration technique is unable to completely recover thrombotic material from most patients with STEMI.
CONCLUSIONSThe main conclusion of this study is that the presence of atheroma plaque fragments in the coronary aspirate of patients with STEMI can provide additional prognostic information and that an immunohistochemical study with CD68 is a good method to detect these fragments. The presence of plaques appears to be associated with a more favorable outcome, independently of other already known risk factors, and plaque absence could be used as a marker for the need for more intensive preventive strategies involving antiplatelet and anticoagulant drugs.
- -
The relationship between coronary aspirate histology and the outcomes of STEMI patients has only been analyzed in 1 previous study, with thrombus age identified as a predictor of mortality.
- -
In published series, more than a third of aspirated samples contain plaque fragments; however, there is little information on the factors determining their presence and whether they are related to prognosis.
- -
The main contribution of this study is that the presence of atheroma plaque fragments in the coronary aspirate of patients with STEMI can provide additional prognostic information.
- -
Immunohistochemical staining with CD68 is a good method to detect these fragments.
None declared.
This work was possible thanks to the help of the catheterization nursing staff and cardiology physicians of our center who participated in the collection of patients’ medical information. We especially thank Diego García Fresnadillo, a technician from the anatomy and pathology laboratory, for sample processing.