There is little information on the effect of acute coronary syndrome complicated by ventricular fibrillation on the long-term incidence of sudden cardiac death. We analyzed this effect in a contemporary cohort of patients with acute coronary syndrome.
MethodsWe studied 5302 consecutive patients with acute coronary syndrome between December 2003 and December 2012. We compared mortality during and after hospitalization according to the presence or absence of ventricular fibrillation.
ResultsVentricular fibrillation was observed in 163 (3.1%) patients, and was early onset in 72.4% of these patients. In-hospital mortality was 36.2% in the group with ventricular fibrillation and 4.7% in the group without (p<.001). After a mean follow-up of 4.7 years (standard deviation, 2.6 years), mortality was 30.7% in the ventricular fibrillation group and 24.7% in the other group (P=.23). After adjusting for confounding variables, the presence of ventricular fibrillation was not associated with an increased risk of death in the follow-up period (hazard ratio=1.29; 95% confidence interval, 0.90-1.87). The cause of death was established in 72% of patients. The incidence of sudden death was 12.9% in the ventricular fibrillation group and 11.9% in the other group (P=.71). Cardiovascular-cause mortality was also similar between the 2 groups (35.5% and 34.4%, respectively.
ConclusionsPatients with acute coronary syndrome complicated by ventricular fibrillation who survive the in-hospital phase do not appear to be at an increased risk of sudden cardiac death or other cardiovascular-cause death.
Keywords
Sudden cardiac death is one of the leading causes of death in Western countries,1,2 and is probably the biggest challenge in cardiology today.
The aim of identifying the possible causes and mechanisms of sudden cardiac death3 is to achieve more effective risk stratification. The leading cause of sudden death is ventricular fibrillation (VF) complicating the acute phase of acute coronary syndrome (ACS).2 Although it has become less common in recent years,2 VF is still a predictor of poor short-term prognosis.4,5 However, the medium- and long-term prognostic influence of VF complicating ACS is less clear, especially in terms of a possible increased risk of sudden cardiac death.4–10 Current guidelines for secondary prevention of sudden death are based on the evidence that the risk of recurrent VF is reduced after acute ischemia has been corrected with revascularization and drug therapy of proven prognostic effectiveness. Therefore, implantable cardioverter-defibrillator (ICD) therapy is not indicated in the early management of patients with ACS complicated by VF.11 However, in recent years the strength of this evidence has been questioned, and authors have suggested that there is still some risk of future fatal arrhythmic events after revascularization and drug therapies.12–14
This study describes the long-term prognosis of patients with ACS complicated by VF and provides data on the incidence of sudden cardiac death in these patients.
METHODSStudy PopulationWe based our retrospective cohort study on all patients entered in the CardioCHUS registry who were consecutively admitted with a diagnosis of ACS to the Cardiology Department of Complejo Hospitalario Universitario de Santiago de Compostela (consisting of the coronary unit, intermediate care, and wards) between December 2003 and December 2012 (n=5640).
We excluded patients with unconfirmed ACS (n=114), and those with ACS triggered by recent surgery, pancreatitis, cholecystitis, substance abuse, severe anemia (hemoglobin<7 g/dL), or sepsis (n=224). Therefore, the final cohort of our study consisted of 5302 patients with a primary, definitive diagnosis of ACS.
We reviewed the medical history of patients with ACS diagnosed with cardiac arrest to identify and include patients diagnosed with VF. The diagnosis of cardiac arrest due to VF was confirmed by the presence of a VF-compatible trace (rapid, disorganized arrhythmia with irregular waves and inconsistent waveform, and QRS complexes indistinguishable from the T waves), and compatible clinical signs and symptoms. We divided the patients into 2 groups by presence or absence of VF, and then subdivided the VF group by early-onset VF (≤ 48 h) and late-onset VF (> 48 h). VF episodes during invasive procedures were not considered VF events in our study.
ACS was classified by acute myocardial infarction (AMI) with ST-segment elevation (STEMI) (ST-segment elevation ≥ 1mm in at least 2 contiguous leads, new or presumed new left bundle-branch block), or non-ST-segment elevation ACS (NSTEACS) (non-ST-elevation AMI and unstable angina). Patients with pacemakers and absence of persistent ST elevation or known left bundle-branch block were classified as NSTEACS.
We measured left ventricular ejection fraction (LVEF) by echocardiography during the hospital stay, using the Simpson rule.
The study was conducted in accordance with the principles of the Declaration of Helsinki.
Objective and Follow-upThe aim of this study was to analyze the prognostic effect of VF on in-hospital mortality, total mortality, and long-term cause of death.
After discharge, patients were followed up at an ischemic heart disease clinic and by their general physicians. Our structured follow-up was based on patients’ unique electronic health record (IANUS program, Galicia Autonomous Community), reviewing all medical contacts and hospital notes. We followed up by telephone in some cases. Mean follow-up was 4.7 years (standard deviation, 2.6 years). We excluded 150 (3%) hospitalized patients (3.8% of the VF group vs 3.0% of the non-VF group; P=.83) from the survival analysis due to missing survival outcome data.
We used the same cause of death classification that we reported in a previous study.15 Sudden death was defined as the unexpected death of a patient who had previously been stable, either in the presence of signs (with or without documented arrhythmia) or in the absence of signs (the patient was seen during the 24hours prior to death, but had shown no signs of heart failure, myocardial infarction, or other clear cause-of-death event). In this study, we considered appropriate ICD shocks triggered by VF as a sudden death event. We defined cardiovascular death as death of cardiac or noncardiac but vascular origin caused by refractory heart failure, acute coronary event, acute aortic syndrome, pulmonary, systemic or cerebral embolism, or renal vascular disease (renal failure in the absence of glomerular disease or other parenchymal abnormality). Other causes of death were considered to be of noncardiovascular origin.
Two cardiologists (N. Bouzas-Cruz and E. Abu-Assi) established the cause of death independently, and in case of disagreement, a third cardiologist was consulted (J.M. García-Acuña). In the absence of consensus, or insufficient information, the patient was included in the “unknown or unclassifiable cause” group.
Statistical AnalysisQuantitative variables were expressed as mean (standard deviation) and qualitative variables as frequencies and percentages. Qualitative variables were compared with chi-square or Fisher exact test.
We used a binary logistic regression model to identify predictors of VF during the first 48hours of admission. We included variables from the univariate analysis that showed an association with P<.10, and variables from previous studies that showed an association with VF within 48hours of the onset of the coronary event, regardless of their statistical significance in the present series. The final logistic regression model to identify predictors of early-onset VF included the following variables: age under 65 years, sex, year of admission, diabetes mellitus, hypertension, history of ischemic heart disease, family history of early ischemic heart disease, previous vascular disease (peripheral arterial disease and/or stroke or transient ischemic attack), chronic obstructive pulmonary disease, previous treatment with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and STEMI vs NSTEACS and anterior wall STEMI.
The influence of factors that showed significant association in the previous analysis with early-onset VF was described using odds ratio (OR) with a 95% confidence interval (95%CI).
Survival curves were then plotted with the Kaplan-Meier method and compared using the log-rank test. We built a Cox regression model to identify mortality-associated factors during follow-up. In the multivariate Cox model, we included variables from the Cox univariate analysis that showed an association, with P<.10. The final model included the following variables: age, sex, history of heart failure, previous vascular disease, diabetes mellitus, hypertension, dyslipidemia, baseline left bundle-branch block or pacemaker, atrial fibrillation, previous AMI, history of cancer, chronic obstructive pulmonary disease, hemoglobin and serum creatinine on admission, admission Killip class>1, ACS type, coronary revascularization and type (percutaneous coronary intervention or surgery) during hospitalization, significant multivessel coronary disease (≥ 70% and ≥ 2 vessels), drug therapy at discharge (aspirin, clopidogrel, beta-blockers, statins, ACE inhibitors/ARBs, loop diuretics, aldosterone antagonists, or digoxin).
Since we did not have LVEF values for all patients (only in 91.8% of cases), we did not initially include LVEF as a covariate in the multivariate Cox model, but later we assessed whether there had been changes in the previous model estimates regarding the prognostic influence of VF after adding the LVEF variable.
The previous analysis was repeated only with patients with early-onset VF to test its influence on prognosis during follow-up.
The P values for statistical significance and the hazard ratio (HR), and their respective 95%CI were calculated and estimated by generating 500 bootstrap replicates.
Statistical analyses were performed with SPSS 21 and STATA 13. Statistical significance was defined as P<.05.
RESULTSVentricular fibrillation was found in 163 patients (3.1%), and was early-onset in 118 (72.4%) of those patients. We observed a nonsignificant downward trend in the annual VF rate from 2004 until 2012 as follows: 3.9%, 3.3%, 3.1%, 3.5%, 3.3%, 2.2%, 2.6%, 2.4%, and 2.9% (Cochran-Armitage test; P=.10). For NSTEACS, the percentage distribution of VF from 2004 to 2012 was 1.3%, 1.1%, 2.3%, 1.0%, 1%, 2.8%, 0.6%, 1.2%, and 0.7%, respectively (P=.56). For STEMI, it was 5.0%, 6.9%, 6.3%, 8.0%, 7.4%, 6.6%, 6.8%, 5.8, and 6.1%, respectively (P=.84).
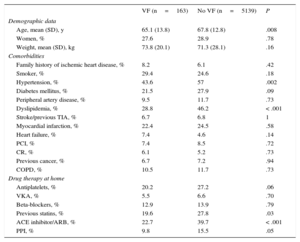
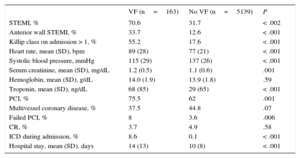
Table 1 shows the baseline characteristics of patients by presence or absence of VF. Patients with VF were significantly younger and had lower prevalence of comorbidities.
Baseline Characteristics of Total Patients by Presence or Absence of Ventricular Fibrillation
| VF (n=163) | No VF (n=5139) | P | |
|---|---|---|---|
| Demographic data | |||
| Age, mean (SD), y | 65.1 (13.8) | 67.8 (12.8) | .008 |
| Women, % | 27.6 | 28.9 | .78 |
| Weight, mean (SD), kg | 73.8 (20.1) | 71.3 (28.1) | .16 |
| Comorbidities | |||
| Family history of ischemic heart disease, % | 8.2 | 6.1 | .42 |
| Smoker, % | 29.4 | 24.6 | .18 |
| Hypertension, % | 43.6 | 57 | .002 |
| Diabetes mellitus, % | 21.5 | 27.9 | .09 |
| Peripheral artery disease, % | 9.5 | 11.7 | .73 |
| Dyslipidemia, % | 28.8 | 46.2 | < .001 |
| Stroke/previous TIA, % | 6.7 | 6.8 | 1 |
| Myocardial infarction, % | 22.4 | 24.5 | .58 |
| Heart failure, % | 7.4 | 4.6 | .14 |
| PCI, % | 7.4 | 8.5 | .72 |
| CR, % | 6.1 | 5.2 | .73 |
| Previous cancer, % | 6.7 | 7.2 | .94 |
| COPD, % | 10.5 | 11.7 | .73 |
| Drug therapy at home | |||
| Antiplatelets, % | 20.2 | 27.2 | .06 |
| VKA, % | 5.5 | 6.6 | .70 |
| Beta-blockers, % | 12.9 | 13.9 | .79 |
| Previous statins, % | 19.6 | 27.8 | .03 |
| ACE inhibitor/ARB, % | 22.7 | 39.7 | < .001 |
| PPI, % | 9.8 | 15.5 | .05 |
ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; CR, coronary revascularization surgery; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; TIA, transient ischemic attack; VF, ventricular fibrillation; VKA, vitamin K antagonist.
Prior to admission, there was a lower rate of treatment with antiplatelets, ACE inhibitors/ ARBs, or statins among patients with VF. There were no significant differences in beta-blocker treatment prior to admission.
Most patients with VF had STEMI. The commonest location was anterior wall. The rate of patients with heart failure on admission was significantly higher among those with VF (55.2% vs 17.6%; P<.001). There were more percutaneous coronary interventions in patients with VF, and a higher percentage of failed procedures among patients with VF than those without (8% vs 3.6%; P=.006). Hospital stay was longer among patients with VF (mean 14 days [standard deviation, 13 days]) than those without (10 days [standard deviation, 8 days]); P<.001 (Table 2).
Admission and In-hospital Management Data, by Presence or Absence of Ventricular Fibrillation
| VF (n=163) | No VF (n=5139) | P | |
|---|---|---|---|
| STEMI, % | 70.6 | 31.7 | < .002 |
| Anterior wall STEMI, % | 33.7 | 12.6 | < .001 |
| Killip class on admission > 1, % | 55.2 | 17.6 | < .001 |
| Heart rate, mean (SD), bpm | 89 (28) | 77 (21) | < .001 |
| Systolic blood pressure, mmHg | 115 (29) | 137 (26) | < .001 |
| Serum creatinine, mean (SD), mg/dL | 1.2 (0.5) | 1.1 (0.6) | .001 |
| Hemoglobin, mean (SD), g/dL | 14.0 (1.9) | 13.9 (1.8) | .59 |
| Troponin, mean (SD), ng/dL | 68 (85) | 29 (65) | < .001 |
| PCI, % | 75.5 | 62 | .001 |
| Multivessel coronary disease, % | 37.5 | 44.8 | .07 |
| Failed PCI, % | 8 | 3.6 | .006 |
| CR, % | 3.7 | 4.9 | .58 |
| ICD during admission, % | 8.6 | 0.1 | < .001 |
| Hospital stay, mean (SD), days | 14 (13) | 10 (8) | < .001 |
bpm, beats per minute; CR, coronary revascularization surgery; ICD, implantable cardioverter-defibrillator; PCI: percutaneous coronary intervention; STEMI, ST-segment elevation acute myocardial infarction; VF, ventricular fibrillation.
In the multivariate analysis, the following factors showed independent association with early-onset VF: age<65 years (OR=1.7; 95%CI, 1.10-2.68), STEMI (OR=6.0; 95%CI, 3.60-9.80), anterior wall STEMI (OR=1.6; 95%CI, 1.07-2.52) and no personal history of ischemic heart disease (OR=2.4; 95%CI, 1.33-4.26).
Discrimination rates (C-statistic=0.79; 95%CI, 0.74-0.83) and model fit were good (Hosmer-Lemeshow test, P=.93).
In-hospital mortality was 5.7% (n=301), with a cardiovascular origin in 82.4% (n=248) of cases.
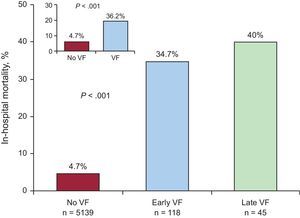
Figure 1 shows in-hospital mortality by presence or absence of VF and by early or late VF subgroup. In the VF group, 59 (36.2%) deaths were registered. In the early VF group, 41/118 (34.7%) died, compared with 18/45 (40%) in the late VF group.
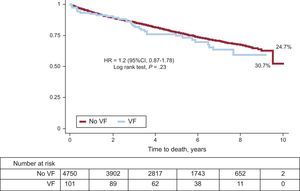
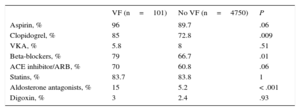
On discharge, significantly more patients with VF were prescribed antiplatelets, beta-blockers, ACE inhibitors /ARBs and aldosterone antagonists (Table 3). During the follow-up (mean, 4.7 years; standard deviation, 2.6 years), 1203 patients (24.8%) died. Figure 2 shows the survival curves by presence or absence of VF during the acute phase. There were no significant differences between groups, although unadjusted total mortality was numerically higher among patients with VF than those without (30.7% vs 24.7%; P=.23).
Treatment on Discharge
| VF (n=101) | No VF (n=4750) | P | |
|---|---|---|---|
| Aspirin, % | 96 | 89.7 | .06 |
| Clopidogrel, % | 85 | 72.8 | .009 |
| VKA, % | 5.8 | 8 | .51 |
| Beta-blockers, % | 79 | 66.7 | .01 |
| ACE inhibitor/ARB, % | 70 | 60.8 | .06 |
| Statins, % | 83.7 | 83.8 | 1 |
| Aldosterone antagonists, % | 15 | 5.2 | < .001 |
| Digoxin, % | 3 | 2.4 | .93 |
ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; VF, ventricular fibrillation; VKA, vitamin K antagonist.
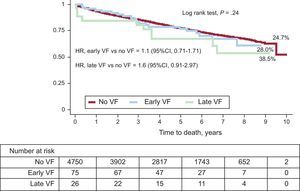
Figure 3 compares the survival curves of patients without VF, with early VF, and with late VF. Mortality rates in these 3 subgroups were 24.7%, 28.0%, and 38.5%, respectively.
During follow-up, an ICD was implanted in 0.9% (42/4851) of patients, representing 5% (5/101) of patients with VF and 0.8% (37/4750) without VF (P<.001).
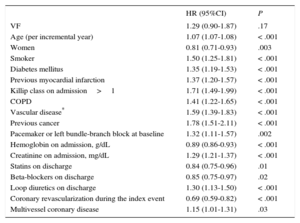
Table 4 shows results after the Cox regression analysis. Ventricular fibrillation was not significantly associated with a higher risk of all-cause death (HR=1.29; 95%CI, 0.90-1.87; P=.17). After adding LVEF to the multivariate survival analysis, VF did not show a significant association with a higher risk of death (HR=1.21; 95%CI, 0.82-1.76; P=.35).
Effect of Ventricular Fibrillation on Long-Term All-Cause Mortality in Index Event Survivors
| HR (95%CI) | P | |
|---|---|---|
| VF | 1.29 (0.90-1.87) | .17 |
| Age (per incremental year) | 1.07 (1.07-1.08) | < .001 |
| Women | 0.81 (0.71-0.93) | .003 |
| Smoker | 1.50 (1.25-1.81) | < .001 |
| Diabetes mellitus | 1.35 (1.19-1.53) | < .001 |
| Previous myocardial infarction | 1.37 (1.20-1.57) | < .001 |
| Killip class on admission>1 | 1.71 (1.49-1.99) | < .001 |
| COPD | 1.41 (1.22-1.65) | < .001 |
| Vascular disease* | 1.59 (1.39-1.83) | < .001 |
| Previous cancer | 1.78 (1.51-2.11) | < .001 |
| Pacemaker or left bundle-branch block at baseline | 1.32 (1.11-1.57) | .002 |
| Hemoglobin on admission, g/dL | 0.89 (0.86-0.93) | < .001 |
| Creatinine on admission, mg/dL | 1.29 (1.21-1.37) | < .001 |
| Statins on discharge | 0.84 (0.75-0.96) | .01 |
| Beta-blockers on discharge | 0.85 (0.75-0.97) | .02 |
| Loop diuretics on discharge | 1.30 (1.13-1.50) | < .001 |
| Coronary revascularization during the index event | 0.69 (0.59-0.82) | < .001 |
| Multivessel coronary disease | 1.15 (1.01-1.31) | .03 |
COPD, chronic obstructive pulmonary disease; HR, hazard ratio; VF, ventricular fibrillation; 95%CI, 95% confidence interval.
When we analyzed patients with early VF alone in the multivariate survival analysis, (n=75), results remained consistent with those observed in the total sample (HR=1.20 (95%CI, 0.77-1.93; P=.39).
We were able to identify the cause of death in 866 (72%) of the 1203 deceased patients. There were no significant differences in cause-specific death by VF and non-VF subgroups (Table 5). Death of cardiovascular cause occurred in 35.5% of patients with VF, vs 34.4% of patients without VF.
Cause of Death by Presence or Absence of Ventricular Fibrillation Among Index Event Survivors
| VF (n=31/101) | No VF (n=1172/4750) | P | |
|---|---|---|---|
| Cardiovascular Death (n=414) | 11 (35.5) | 403 (34.4) | .71 |
| Sudden (n=144) | 4 (12.9) | 140 (11.9) | |
| Not sudden (n=270) | 7 (22.6) | 263 (22.4) | |
| Noncardiovascular Death (n=452) | 10 (32.3) | 442 (37.7) | |
| Unknown or Unclassifiable (n=337) | 10 (32.3) | 327 (27.9) |
VF, ventricular fibrillation.
Data are expressed as No. (%).
Among the patients with ICD, 3 had VF during follow-up. During the acute phase of the ACS, 2 of these patients did not have VF and 1 had a VF episode.
Although the sudden death rate was numerically higher among patients with VF (12.9%), there were no significant differences compared with the non-VF group (11.9%; P=.71). The distribution of death with unknown or unclassifiable cause did not differ significantly between the 2 groups (32.3% vs 27.9%; P=.74).
DISCUSSIONIn this contemporary cohort of patients with ACS, the incidence of VF complicating the course of an acute coronary event is relatively low, and shows a nonsignificant downward trend over the years. Our results show that despite the relatively low frequency of VF, this complication has a very high mortality rate in the acute phase of ACS, especially if it occurs more than 48hours after diagnosis of an acute coronary event. In contrast, total mortality attributable to VF in the acute phase remains unchanged after hospital discharge. Similarly, the risk of death from a sudden cardiac event or other cardiovascular cause is unaltered in the long-term follow-up.
Ischemic heart disease is the main cause of sudden death in Western countries, representing 75% of all cases.1 Acute myocardial ischemia is the leading cause of VF.2 The increased risk of fatal arrhythmic events mainly occurs in the first weeks after the acute event, and it then decreases substantially, leveling out about 1 year later.9
Ventricular fibrillation is found in 2% to 8% of patients with ACS.2,4–10 Although these patients can be considered to be at high risk of recurrent VF in the case of a new ischemic episode or during the course of the disease, no randomized clinical trials have been conducted on the potential benefit of ICD in this patient population. Furthermore, studies that have investigated this little-known area have found a varied prognosis for patients with ACS complicated by VF, ranging from poor in some series4,5 to excellent in others.6–8
Current clinical guidelines do not recommend an ICD as secondary prevention in patients with AMI complicated by VF in the first 48hours postdiagnosis, in the absence of residual ischemia or severe ventricular dysfunction.11 It is important to note that these class C recommendations are based on 2 very old studies,16,17 and therefore the validity of conclusions drawn from these studies may now be questionable. Furthermore, clinical, epidemiologic, and genetic studies have found that certain individuals are at a high risk of VF recurrence.12–14 These patients may indeed have a repeat episode of VF should they develop a new ischemic event, and in fact such an event is usually the main trigger of VF.2
Therefore, taking into account all the above evidence, studies that investigate the influence on long-term prognosis of ACS complicated by VF may be of particular clinical relevance. Our study represents the largest series to date of patients with VF as a complication of ACS.
Among the most up-to-date series, including those analyzed by Piccini et al,5 Bougouin et al,10 and de Jong et al,8 our study is the most recent, and therefore it is likely that it more faithfully reflects current ACS management and treatment protocols, which have led to considerably improved prognoses in this clinical scenario. Our series included AMI patients with and without ST-segment elevation, unlike the studies by Piccini et al and de Jong et al, which only included patients with non-ST-elevation AMI and STEMI, respectively.
In addition, Piccini et al based their study on a careful selection of patients from the EARLY ACS trial.5 Their substudy investigated the prognostic effect of sustained VF or ventricular tachycardia in 228 patients with non-ST-elevation AMI. The authors found that 30-day mortality was almost 6 times higher in the sustained VF or ventricular tachycardia group, and that in-hospital mortality was particularly high among patients presenting with late-onset arrhythmic events (≥ 48hours). The authors also found that the higher risk of death among these patients persisted at 1 year. However, the study did not provide information on the risk of sudden death after discharge.
Bougouin et al10 studied 116 patients with VF and AMI (with and without ST-elevation). They observed that all-cause death and cardiac-specific sudden and non-sudden death (mean, 4.3 years after discharge; standard deviation, 0.17 years) did not significantly differ according to the presence or absence of VF.
Finally, de Jong et al8 studied 341 patients with a first STEMI and primary VF. The authors found that all-cause mortality was 6.6% in the VF group vs 9% in the non-VF group (P=.106). They did not determine cause-specific mortality.
Therefore, our findings on the long-term prognostic influence of ACS complicated by VF confirm the conclusions drawn by Bougouin et al and de Jong et al regarding unchanged risk of all-cause mortality.8,10
Our data also confirm that younger age10,17 and STEMI4 are predictors of early-onset VF in ACS. Unlike other studies that identified inferior STEMI as a risk factor for developing early VF,6 our series identified anterior STEMI as a risk factor. Notably, in a study of over 9000 patients with AMI, only a posterior location showed a protective effect against VF, compared with other locations.
Finally, the high in-hospital mortality of patients with VF in our series coincides with observations in other studies.4,5,10
Our findings may have clinical relevance, because these patients were not specially selected and they were treated according to current guidelines. Our study validates the recommendations not to perform ICD implantation in all patients with ACS complicated by VF to prevent a possible future risk of sudden cardiac death.11 Also, our conclusion that anterior wall STEMI entails a higher risk of early VF emphasizes the importance of electrocardiographic (ECG) monitoring during hospitalization in the subgroup of patients at high risk of early VF. Current guidelines recommend ECG monitoring of patients with AMI for at least 24hours post-event, and for 72hours in patients at high risk of complex arrhythmias.18,19
LimitationsOur study was retrospective, and has the limitations inherent in this type of design. We only included patients discharged from our department, but due to the local ACS management policy at our institution we believe that very few patients were admitted to other departments, so it is unlikely that this had a significant impact on the results. Although the diagnosis of VF was based on the ECG and clinical context, in view of the retrospective nature of the study, some cases may have been diagnosed incorrectly. This is very unlikely, because the definitive diagnosis was established by at least one cardiologist, and sometimes another cardiologist's opinion was sought during the hospital stay. Also, the diagnosis was confirmed on discharge. In addition, the cause of death could not be established in 28% of patients and data on survival outcome was not collected in 3%. Another limitation of our study is the missing information on LVEF in some patients. Also, LVEF was measured early (during the hospital stay), and therefore its effect on the statistical analysis may be misleading. Due to the very low number of sudden deaths among patients with VF, we were unable to perform an analysis stratified by early/late VF subgroups.
Finally, we were unable to analyze sustained ventricular tachycardia separately; therefore, our findings should be interpreted in this context and compared with other studies that analyze VF and sustained ventricular tachycardia together and separately. It has recently been observed that ventricular tachycardia alone or ventricular tachycardia/VF in patients with AMI is not associated with a higher risk of death over a 5-year follow-up.10 In addition, VF is often triggered by acute myocardial ischemia,20 while ventricular tachycardia generally has a structural origin.21,22 The fact that our multivariate survival analysis included several factors that generally have a structural component helps to reduce the above limitation of our study.
CONCLUSIONSIn this contemporary patient cohort, VF was relatively uncommon in the acute phase of ACS. Ventricular fibrillation mainly occurred during the first hours of the event and was associated with very high in-hospital mortality. However, in these patients, VF did not imply an increased risk in all-cause death or sudden cardiac death after discharge. This finding strengthens current indications and recommendations in clinical practice guidelines regarding the need to recommend ICD implantation in these patients to prevent the potential risk of recurrent VF, either in the context of a new ACS or as part of the chronic course of heart disease itself.
CONFLICTS OF INTERESTNone declared.
We are grateful to all the health care staff and other staff at the Servicio de Cardiología del Hospital Clínico Universitario de Santiago de Compostela for their exceptional clinical work and for seeking excellence in health care. We would like to offer particular thanks to the Critical Care Unit nurses Isabel Arufe and Belén Outes who participated in this study.