Hybrid imaging for ischemic heart disease refers to the fusion of information from a single or usually from multiple cardiovascular imaging modalities enabling synergistic assessment of the presence, the extent, and the severity of coronary atherosclerotic disease along with the hemodynamic significance of lesions and/or with evaluation of the myocardial function. A combination of coronary computed tomography angiography with myocardial perfusion imaging, such as single-photon emission computed tomography and positron emission tomography, has been adopted in several centers and implemented in international coronary artery disease management guidelines. Interest has increased in novel hybrid methods including coronary computed tomography angiography-derived fractional flow reserve and computed tomography perfusion and these techniques hold promise for the imminent diagnostic and management approaches of patients with coronary artery disease. In this review, we discuss the currently available hybrid noninvasive imaging modalities used in clinical practice, research approaches, and exciting potential future technological developments.
Keywords
Invasive and primarily noninvasive cardiac imaging play a key role in modern cardiology for the assessment and further management of patients with coronary artery disease (CAD). In the last decade, unprecedented advances have been made in several noninvasive cardiac imaging technologies that have altered the diagnostic approach to CAD patients. Improvements in hardware and software as well as the introduction of new hybrid scanners have allowed the performance of hybrid imaging in ischemic heart disease,1 which is nowadays relatively widely adopted and also implemented in international guidelines.2–4
Ischemic heart disease refers to a composite entity that encompasses anatomical alterations of the coronary arteries as well as functional consequences for the heart muscle. Individual cross-sectional cardiac imaging modalities provide general information primarily on one of those aspects. Physicians have long mentally fused information from different modalities that, combined with the clinical status of the patient, has allowed them to decide on the diagnostic approach and the most appropriate therapeutic management. In the era of multimodality imaging, the advent of hybrid imaging has eased this mental fusion of the wealth of information available.5 Coronary artery anatomy and coronary plaque morphology is readily provided by coronary computed tomography angiography (CCTA) while myocardial perfusion imaging (MPI), including single-photon emission computed tomography (SPECT), positron emission tomography (PET) and cardiac magnetic resonance imaging (MRI), can identify regional myocardial perfusion defects, thereby indicating hemodynamically significant lesions. The combination of this information could minimize the percentage of false negative and false positive results of each modality, thereby increasing our diagnostic accuracy.
In this review, we discuss the current available hybrid noninvasive imaging modalities implemented in clinical practice, research targets, and potential novel technological developments.
CLINICAL BACKGROUNDIt is well established that the anatomical severity of coronary stenoses (as measured by invasive coronary angiography or by CCTA) does not correlate well with the presence/extent of myocardial ischemia. Furthermore, the use of mainly anatomical criteria for revascularization in patients with stable ischemic cardiac disease has not been demonstrated to improve survival.6 Elegant prospective studies have demonstrated the superiority of revascularization over optimal medical management for patients with hemodynamically significant coronary lesions.7–10 and functional tests for myocardial ischemia have been incorporated into the revascularization guidelines.2 Invasive fractional flow reserve (FFR) was introduced more than 2 decades ago and has subsequently been used as the reference standard to assess lesion-specific ischemia.11 In patients with multivessel CAD, this functional test used to guide revascularization significantly reduces mortality and myocardial infarction at 2 years when compared to standard angiographic anatomic measurements alone.12
As it is evident that not all patients referred for suspected CAD or control of the progression of the disease should undergo invasive assessment, noninvasive anatomical and/or functional tests remain the draught horse of our diagnostic approach. Commonly, a single modality–CCTA or functional testing–may suffice for the majority of patients with low-to-intermediate pretest likelihood.13,14 Nevertheless, in some patients, imaging results may be inconclusive and the addition of a second test will increase diagnostic accuracy and might assist the further medical and/or interventional management of the patient. Certainly, increased costs are a significant factor to consider as is additional radiation, although there has recently been an impressive radiation dose reduction in both CCTA systems15 as well as in MPI approaches.16,17
TECHNICAL BACKGROUNDHybrid imaging for ischemic cardiac disease can refer either to fusion of more than one imaging modalities or to combining information on both physiology and anatomy derived from a single modality. The multimodality approach has been in use in the cardiac field for more than a decade now,18 whereas single modality techniques have been developed and tested lately, such as computed tomography-derived estimation of FFR (CT-FFR)19 and computed tomography perfusion (CTP).20
Hybrid scanners combining newer generation CCTA scanners with SPECT, PET, or MRI are now relatively readily available from several manufacturers.21 Technological leaps, such as the introduction of solid-state semiconductor detectors with multipinhole collimators in SPECT cameras, have allowed short acquisition times (circa 5minutes), reducing radiation dose and improving diagnostic accuracy.16,22 In principle, most hybrid approaches require that the patient is sequentially imaged in different scanners or in the same scanner. However, newer systems such as PET/cardiac MRI also allow simultaneous acquisitions of signal with both modalities and open new avenues for research and clinical applications.
Similarly to hardware improvement, hybrid dedicated software options are provided by manufacturers enabling the realization of the full potential of hybrid imaging in CAD.18 These software packages, following automated/semiautomated segmentation, generate 3-dimensional (3D) reconstructions of myocardial perfusion data that are fused with 3D coronary anatomy datasets resulting in a hybrid 3D visualization. Furthermore, most of the novel SPECT/CT systems provide newer image reconstruction techniques, such as iterative reconstruction algorithms that allow for improved count sensitivity and enhance image quality.23 A key element of these software packages is the ability to accurately coregister the different imaging data, these being anatomical information on coronary anatomy or ventricle morphology, with functional data such as those obtained from radionuclide studies. Accounting mainly for cardiac and respiratory motion as well as for the intrinsic mismatch in the size and shape of the left ventricle among diastolic ECG-gated CCTA and the nongated SPECT images, manual superposition and individual correction of data set misalignment is helpful to ensure adequate quality.
Aside from the above-mentioned technologies, newer advances in computational approaches have allowed the estimation of hemodynamic parameters from cross-sectional imaging modalities, such as CT-FFR,19 endothelial shear stress and wall stress.24 The majority of these have been primarily used in research, with the exception of CT-FFR, which has been clinically validated vs invasive FFR.25,26 Currently proprietary software19,27 is in use, allowing for simulation of hyperemia from static CCTA images, while more recently open source options28 have been developed, paving the way for more widespread adoption.
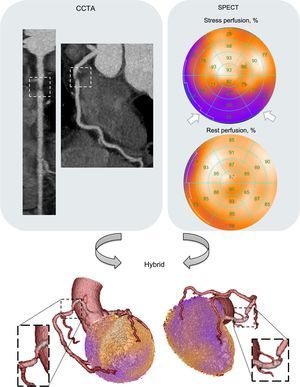
CLINICAL APPLICATIONSCoronary Artery DiseaseAs previously mentioned, in the patient population with low pretest-probability, CCTA alone may be sufficient to rule out epicardial CAD. However, cardiac patients with intermediate pretest-probability are those that are expected to benefit the most from hybrid approaches (Figure 1). To this end, hybrid imaging has been shown to provide added diagnostic information for culprit lesion identification and for guiding target vessel revascularization. Fusion of functional and anatomical information enables noninvasive comprehensive CAD assessment by allowing exact matching of perfusion defects to the corresponding subtending coronary artery, which may help to avoid unnecessary invasive angiographies and revascularization procedures.29 Several early small studies have supported the added clinical value of hybrid imaging in ischemic cardiac disease.29,30
Characteristic example of a coronary computed tomography angiography (CCTA)/single-photon emission computed tomography-myocardial perfusion imaging (SPECT MPI) hybrid study. A highly stenotic plaque in the proximal right coronary artery (insert) depicted by CCTA accurately matches a myocardial perfusion defect in the inferior wall (white arrows) assessed by a SPECT MPI perfusion test.
In a multicenter clinical trial, including 252 patients with stable angina and intermediate pretest CAD probability who underwent MPI, CCTA and invasive FFR measurement,31 hybrid imaging was shown to reliably allow colocalization of myocardial perfusion defects with subtending coronaries compared with standardized myocardial segmentation models on account of variations in individual coronary anatomy. In two-thirds of the patients with an intermediate pretest probability of CAD, hybrid imaging was suggested to efficiently provide a noninvasive ‘rule-in/rule-out’ algorithm of patients with hemodynamically significant CAD. Another single-center prospective clinical study involving 208 patients with suspected CAD who underwent CCTA, technetium 99m/tetrofosmin SPECT, and [15O] H2O PET revealed that PET yielded the highest diagnostic accuracy to detect myocardial ischemia vs invasive FFR.32 Despite a rather high prevalence of disease, the authors did not observe improved accuracy for combined physiological and anatomical imaging by hybrid methods.33 By contrast, a recent meta-analysis of 12 studies, showed that hybrid cardiac imaging improved diagnostic specificity for the detection of obstructive CAD compared with stand-alone coronary CTA, but with limited improvement in overall diagnostic performance.5 Regarding the prognostic implications of hybrid imaging in a series of 324 patients, a matched defect on a hybrid image was found to be a strong predictor of major adverse cardiovascular events during a median follow-up of 2.8 years.34
Chronic Total OcclusionsInterventional management of chronic total occlusions is related to higher rates of complications and patient exposure to higher radiation doses, given the time required under fluoroscopic guidance.35 In these patients, documentation of myocardial viability and/or ischemia is considered necessary prior to complex percutaneous interventions. Hybrid imaging can provide anatomical and functional information on the extent of the diseased myocardium and morphological characteristics of the occluded coronary segment. The latter include the length and the course of the occluded arterial segment, the artery size and the presence of calcifications, among other characteristics, and are considered to be predictors of the procedural success of revascularization procedures.21,36 Ideally, preinterventional imaging (mainly with CCTA) should be considered for complex chronic total occlusions with an expected success rate < 50% and in cases of reinterventions following initial chronic total occlusion recanalization failures.37,38
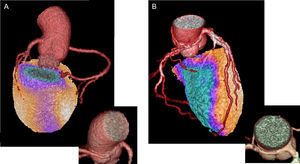
Coronary Anomalies/Multivessel Disease/Side BranchesCoronary artery anomalies represent a rather rare clinical entity, but nevertheless are increasingly recognized given the widespread use of noninvasive cardiac imaging.39 Although most are characterized as benign in terms of hemodynamic significance and remain undiagnosed, those that are associated with inducible myocardial ischemia might have significant therapeutic consequences.40 Cardiac imaging plays a key role in the diagnostic approach to these patients, with the variant of anomalous coronary artery from the opposite sinus (ACAOS) being best studied, first and foremost with the use of hybrid imaging. Single-photon emission computed tomography MPI and PET MPI combined with CCTA may unmask ischemia in asymptomatic and symptomatic patients and will indicate the nature of the coronary anomaly. In a single-center study including 46 patients with coronary anomalies and possible copresence of CAD, among which 26 (57%) were ACAOS, hybrid CCTA and SPECT MPI was shown to be a valuable noninvasive tool to distinguish the impact of ACAOS from concomitant CAD on myocardial ischemia.41 It was demonstrated that, in a middle-aged population, myocardial ischemia is more likely attributable to concomitant CAD. Furthermore, in a smaller group of middle-aged patients with complex coronary anomalies, hybrid CCTA and PET MPI perfusion defects as assessed by semiquantitative PET MPI were rare and attributable to concomitant CAD rather than to the anomalous vessel itself.42 In contrast, pathological myocardial blood flow, assessed by quantitative hybrid CCTA PET MPI, was seen in the majority of patients in the vessel territories subtended by the anomalous coronary artery. Fused hybrid CCTA/PET MPI incorporating information on morphology and on semiquantitative and quantitative myocardial perfusions may provide added value for the management of patients with coronary anomalies (Figure 2).
Usefulness of hybrid imaging in patients with anomalous coronary artery from the opposite sinus. A: fused coronary computed tomography angiography (CCTA) and single-photon emission computed tomography-myocardial perfusion imaging (SPECT MPI) images of a 44-year-old man with a malignant anomalous right coronary artery from the left coronary sinus and with a slit-form interarterial course. The CCTA revealed the anomalous origin and course of the artery as well as the absence of concomitant coronary artery disease. The SPECT MPI stress images under supramaximal stress testing revealed an inferior wall myocardial perfusion defect. Using hybrid imaging, the defect was shown to belong to the myocardium area subtended by the anomalous artery. Panel B similarly shows the hybrid CCTA and positron emission tomography-myocardial perfusion imaging images of an anomalous right coronary artery of a 73-year-old man, in this case with the concomitant presence of extensive coronary artery disease. The subtended myocardial ischemic territory was matched in the hybrid images with the course of the anomalous right coronary artery.
Apart from anatomical variants, most of the patients with multivessel disease often have less hemodynamically significant lesions than might be presumed from their coronary angiographies.43 In this respect and because they constitute a growing CAD patient population, hybrid imaging may represent an incremental diagnostic tool to assess the flow-limiting plaques that would actually justify a targeted revascularization procedure.21 Similarly, in lesions involving side branches, hybrid imaging can enhance our interventional management by accurately coregistering smaller perfusion defects to the culprit smaller branches (such as diagonal, or marginal branches).
Calcium Scoring CT Scans and SPECT MPIBesides high-end hybrid modalities, the combination of native coronary artery calcium scoring-CT scans with SPECT MPI represents a simpler hybrid approach that is easier to implement into clinical practice, since many centers already perform coronary artery calcium scanning on a large scale either as part of a formal CCTA or as a screening routine examination. Even older-generation scanners such as 64-slice CCTAs have been shown to have good agreement with invasive coronary angiography and this agreement was independent of the calcium score.44
These low-dose native scans performed on a stand-alone CT scanner can be also used for attenuation correction of the SPECT MPI images. The added value of even this mode of anatomical information on the presence, extent and localization of coronary calcium has been shown in a series of 77 patients, in whom the addition of this information to SPECT-perfusion imaging significantly enhanced the diagnostic accuracy of angiographically-significant CAD compared with SPECT alone.45 The SPECT MPI test results together with calcium score were shown to be strong preoperative risk predictors, as evidenced by a study in 326 patients prior to noncardiac surgery.46 Calcium score can allow for further risk stratification, with a very low risk when the score is < 1314 and with normal SPECT MPI findings. In addition, an abnormal SPECT MPI test and a score of ≥ 1314 has added value in predicting adverse outcome.
Further improving the interpretation of a SPECT MPI test, high atherosclerotic burden as assessed by calcium score in patients with suspected CAD and a normal perfusion test might indicate the presence of balanced ischemia by multivessel disease. On the other hand, an equivocal or mildly positive SPECT-perfusion test in an asymptomatic patient with no presence of coronary calcification might be indicative of artifacts.47 Last, this hybrid modality was shown to have added prognostic value in allocating calcified coronaries to their respective myocardial subtended territories while a matched defect was revealed to be an independent predictor of major events in a median follow-up of 6.9 years.48 Given these favorable results from studies investigating the applicability and prognostic value of this attractive, definitely less expensive hybrid approach, more cost-effectiveness studies are warranted to determine whether SPECT MPI plus calcium scoring modalities can represent the first line imaging test for the low and intermediate risk population.
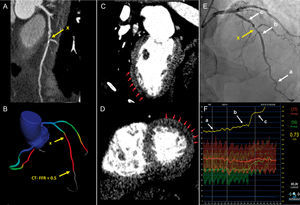
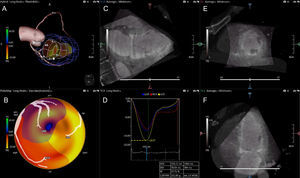
CT Perfusion and CT-FFRThe use of CCTA as a one-stop-shop, integrating myocardial function or lesion-specific hemodynamic information with coronary anatomy, CTP,49,50 and CT-FFR51 have both been investigated in a series of studies for ischemia detection in patients with known CAD and in patients with suspected CAD, respectively.52 Computed tomography perfusion can provide us with information on myocardial blood flow based on the amount of iodine contrast in the myocardium, whereby areas of hypoattenuation correspond to perfusion defects. Computed tomography-derived estimation of FFR can address the hemodynamic significance of epicardial coronary lesions53 (Figure 3). As evident by the original reports and by several meta-analyses, both hybrid modalities may improve the specificity and positive predictive value of CCTA for lesion-specific ischemia without affecting the high sensitivity of the test to detect the disease.54–56 Computed tomography perfusion and CT-FFR are most likely here to stay, and will enjoy their position in the diagnostic approach of the patient with ischemic cardiac disease, once the current limitations for its widespread adoption, including high radiation for CTP and high costs for CT-FFR, are addressed.
Clinical case example of computed tomography-derived estimation of fractional flow reserve (CT-FFR) and computed tomography perfusion (CTP) in a patient with significant stenosis on coronary computed tomography angiography. A 65-year-old man presenting with chest pain and suspected coronary artery disease. A: coronary computed tomography angiography demonstrated significant stenosis (yellow arrow, x) in the proximal LAD. B: CT-FFR calculated at the distal portion of the vessel was < 0.5, indicating functionally significant disease. C and D: visual assessment of CTP-identified perfusion defects in the mid to apical anterior and antero-lateral segments (red arrows) corresponding to ischemia in the LAD territory. E: invasive coronary angiography demonstrated a moderate 50% to 60% proximal stenosis in the LAD (yellow arrow, x). F: invasive FFR measurement showed functionally significant disease at 0.73 in the distal vessel (A) with the largest gradient from (B) to (C) across the lesion. LAD, left anterior descending coronary artery. Reprinted with permission from Schuijf JD et al.52
The combination of PET and MRI has been the focus of attention recently with small pilot studies emphasizing the ability of the modality to provide accurate anatomic plus advanced soft tissue contrast information and insight into plaque biology, primarily in terms of the presence of inflammation.57 Recent technological advances58 with the development of avalanche photodiode and silicon photomultiplier detectors have enabled the performance of simultaneous image acquisition in hybrid PET/MRI scanners. Driven by the low radiation with the combination of these 2 modalities, feasibility studies have been undertaken in the field of ischemic cardiac disease, using mainly 18F-fluorodeoxyglucose (18F-FDG) and 18F-fluoride59,60 as PET tracers.
Both modalities individually have been in use for the assessment of myocardial perfusion and viability in hibernating myocardium. Such hybrid approaches not only allow for concurrent anatomical and metabolic milieu assessment but also synergically compensate one another with regards to the limitations of each modality.61 Recent studies including a small number of postinfarct patients and hybrid PET/MRI were able to show close agreement between the area-at-risk, indicated by 18F-FDG, and MR T2-mapping,62 while late gadolinium enhancement transmurality and 18F-FDG performed equally well in predicting myocardial functional recovery.63 More specifically, the extent of myocardium as assessed by both modalities was shown to have predictive value for regional wall motion improvement in the subacute phase after myocardial infarction. Nevertheless, these small studies have also raised concerns about the usefulness of such costly hybrid modalities in the clinical setting. As in the other hybrid imaging modalities, the actual value of PET/MRI in supporting clinical decision-making for the patient with stable CAD warrants larger data from purpose designed studies.
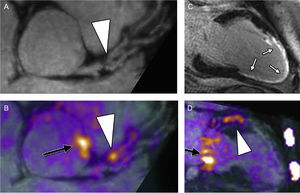
Imaging of the atherosclerotic process with PET/MRI has been demonstrated in large-diameter arteries including carotids60,64 and the aorta.65 Reliable assessment of further vascular beds, such as the coronaries, remains challenging due to the smaller dimensions and the additional problems of respiratory- and cardiac motions-derived artifacts. The latter issue was successfully addressed in a feasibility study using PET/MRI and 18F-fluoride.59 By implementing a free-breathing, motion-insensitive MR attenuation correction map, 18F-fluoride hotspots were identified within the coronary arteries in 7 patients (Figure 4). In theory integration of molecular, functional, and morphological imaging is expected to improve our understanding of the pathobiology of atherosclerotic plaques and the natural history of high-risk/event prone plaques.66
Hybrid PET/MRI. Reformatted views of a culprit plaque in the left anterior descending coronary artery of a patient at 6 months after myocardial infarction: plaque (arrowhead) is observed, causing a proximal luminal stenosis on magnetic resonance imaging (MRI) angiography (panel A). Increased 18F-sodium fluoride (NaF) uptake is observed at exactly this site on fused positron emission tomographic (PET)/MRI (panel B, arrowhead). An extensive near-transmural myocardial infarction is confirmed on late gadolinium enhancement images corresponding to the perfusion territory of this lesion (white arrows) (panel C). Elevated 18F-NaF uptake is again observed in the culprit lesion in the left anterior descending coronary artery on a 2-chamber view of the left ventricle (arrowhead, panel D). Also note increased uptake in the aortic root (black arrow, panel B) and in the mitral valve annulus (black arrow, panel D). All images were acquired during a single PET/MRI scan. Reprinted with permission from Robson et al.59
The low radiation, highly important in young patients, and the synergistic information that can be obtained render PET/MRI highly attractive; nevertheless, the incremental value of the modality against existing techniques remains to be demonstrated. Further technological leaps such as overcoming attenuation correction issues (with the first experience already reported)67 as well as larger feasibility and cost-effectiveness studies are warranted for this cardiac imaging modality to enter the clinical arena. Most certainly, PET/MRI can open fascinating new research horizons for ischemic cardiac disease with regards to disease pathophysiology and natural history.
3D Echocardiography and CCTARecently, the fusion of CCTA and 3D speckle-tracking stress and rest echocardiography has gained attention.68,69 Like the fusion of other modalities with CCTA, hybrid CCTA/3D echocardiography allows direct visualization of the coronary arteries and myocardial strain in the subtended territories (Figure 5). This concept was evaluated in 28 patients with chest pain with a dedicated software for the fusion of CCTA and 3D echocardiography data and using CTP as the reference standard for the detection of ischemia.69 Three-dimensional echocardiography-derived strain abnormalities were defined qualitatively by visual assessment of the color-coded strain maps, while the coronary anatomy and course were extracted from the CCTA. The fusion of resting strain information from 3D echocardiography with anatomic information from CCTA might provide an alternative inexpensive, and safe (low radiation exposure), option for the assessment of patients with suspected CAD.
Three-dimensional (3D) echocardiography and coronary computed tomography angiography (CCTA) fusion. A: CCTA derived coronary anatomy fused with the 3D echocardiography-derived wall motion tracking image of the left ventricle. B: polar map of longitudinal strain (LS) based on CCTA derived coronary anatomy. D: LS curves for each coronary vascular territory. C, E and F: CCTA multiplanar reconstruction planes with 3D echocardiography overlay. Ant, anterior; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; HLA, horizontal long axis; inf, inferior; LAD, left anterior descending; lat, lateral; LCX, left circumflex; post, posterior; sept, septal; RCA, right coronary artery; SA, short axis; VLA, vertical long axis. Reprinted with permission from de Knegt et al.68
Within the broader term of hybrid imaging research, efforts also include those employing information on the cardiac and coronary anatomy with computational simulation of cardiac motion and coronary blood flow. Elegant research has provided us with insights on the pathobiology of plaque formation by assessing morphological plaque characteristics combined with information on the local hemodynamic environment. Endothelial shear stress studies in animals and humans have been performed largely in combination with intravascular coronary imaging,70–73 whereas the latest reports have incorporated noninvasive endothelial shear stress calculations from CCTA.74 Evaluation of hemodynamic and geometric indices,24 such as endothelial shear stress and wall stress, with the aid of hybrid imaging is expected to advance our understanding of the natural history of ischemic cardiac disease and might potentially improve contemporary management approaches.
CONCLUSIONSHybrid cardiac imaging has entered the clinical arena and is increasingly being implemented in the diagnostic approach of patients with ischemic cardiac disease. Undoubtedly, more studies are warranted to assess all clinical aspects such as the effect on management decisions, improvement of outcomes, and cost/effectiveness that would justify the increased radiation exposure from several currently-available hybrid approaches. Combining functional with morphological information on the presence, extent and severity of the disease can facilitate patient-tailored risk stratification and management.
CONFLICTS OF INTERESTNone declared.