Postacute COVID syndrome (PACS) is common after acute SARS-CoV-2 infection. One of the most frequent and disabling symptoms is exercise intolerance (EI). Recent evidence suggests that EI in PACS has a peripheral (metabolic-neuromuscular) origin, suggesting that exercise training may be an effective treatment. The aim of this study was to assess the role a therapeutic physical exercise program (TPEP) in PACS with EI.
MethodsThis single-center, open-label, randomized clinical trial compared an exercise training program (intervention group) with regular physical activity recommendations (control group) in patients with PACS and EI. The intervention group underwent an 8-week TPEP. The primary endpoint was improvement in functional capacity, assessed as the change in peak VO2.
ResultsWe included 50 participants with PACS (73% women, mean age 47±7.1 years). The intervention group showed a 15% improvement in peak VO2 (peak VO2 pre- and postintervention: 25.5±7.7mL/kg/min and 29.3±4.7 mL/kg/min; P <.001) and a 13.2% improvement in predicted values (92.1±14.3% and 108.4±13.4%; P <.001). No significant changes in VO2 values were observed in the control group. Unlike the control group, the intervention group also showed improvements in all secondary outcomes: quality of life scales, muscle power, maximum inspiratory power, metabolic flexibility, and body fat percentage.
ConclusionsThe program improved functional capacity in patients with PACS and EI.
Keywords
Since the beginning of the COVID-19 pandemic, more than 13 500 000 people have been infected with SARS-CoV-2 in Spain.1 Between 10% and 30% are estimated to have persistent symptoms after the acute phase.2–4 Postacute COVID syndrome (PACS) is defined as symptom persistence for more than 12 weeks after infection5 and its development is not related to symptom severity. Exercise intolerance (EI), defined as dyspnea on exertion or marked asthenia, is one of the most frequent symptoms of PACS and is associated with reduced quality of life.6
From the onset of the pandemic, a heterogeneous cardiorespiratory impact was recorded, with symptoms ranging from mild to severe, including pulmonary thromboembolism, myocarditis, and pulmonary fibrosis.7 However, the vast majority of patients with PACS and EI but without severe symptoms show no cardiorespiratory abnormalities during follow-up.8–11
For the functional assessment and study of dyspnea and EI, the gold standard is cardiopulmonary exercise testing (CPET). By using CPET, our group was the first to identify a peripheral origin for EI in PACS.11 A recent meta-analysis of our work and 37 other studies conducted using CPET and including more than 2000 patients with PACS strengthened the hypothesis of defective peripheral oxygen uptake as one of the possible causative mechanisms of the EI.9 One of these studies8 used invasive measurement of O2.
Physical exercise is the most effective way to boost peripheral oxygen uptake, by improving metabolic and neuromuscular function. In addition, various observational studies have proven that exercise-based rehabilitation improves peak VO2 and strength in patients with PACS.12 However, no randomized study has thus far used CPET to assess the impact of a therapeutic physical exercise program (TPEP) in patients with PACS and EI. The objective of the present RECOVER study (Readaptación funcional basada en ejercicio físico en pacientes con COVID persistente [Functional rehabilitation based on therapeutic exercise training in patients with postacute COVID syndrome]) was thus to assess the efficacy of a TPEP in patients with PACS and EI.
METHODSStudy designThis single-center, open-label, randomized clinical trial compared a TPEP (intervention group [IG]) with the exercise recommendations of current clinical practice guidelines13 (control group [CG]). The protocol was approved by the ethics committee of our center. All patients provided consent.
Patient selectionThe following inclusion criteria were applied: a) adults aged between 18 and 65 years with a history of COVID-19; b) symptoms compatible with PACS more than 12 weeks after infection, including asthenia and dyspnea on exertion; c) no previous symptoms; d) absence of another condition explaining the symptoms; and e) provision of signed informed consent. The exclusion criteria were a) physical or psychiatric limitation impeding participation in a TPEP, and b) presence of impediments for completing the TPEP.
Baseline assessment, randomization, and statistical analysisPatients were 1:1 randomized to the IG or CG. We then performed CPET, measurement of maximal inspiratory pressure, body composition analysis using bioimpedance, neuromuscular assessment with load-velocity profiles determined using linear encoders for various muscle groups, and evaluation using the following scales: Post-COVID-19 Functional Status (PCFS), EuroQol scale (EQ-5D-5L), and Patient Health Questionnaire-9 (PHQ-9).
Sample size was calculated using a t test with a 2-tailed significance level of 5% based on previous studies of PACS showing a decreased VO2 and other CPET studies in another population type that determined that TPEP improved the peak VO2 by about 12%.14 Eighteen patients were required in each group (power > 80%).
For quantitative variables, a descriptive analysis was conducted using mean ± standard deviation or median [interquartile range], according to distribution. Qualitative variables are reported using frequency and percentage. The normality of the distribution was tested using the Kolmogorov-Smirnov test. Continuous variables were compared using a t test or Mann-Whitney U test. For qualitative variables, a chi-square test or Fisher test was used. In all analyses, a 2-tailed P value < .05 was considered statistically significant. Data analysis was performed via an intention-to-treat analysis, according to randomization. The analytical software used was STATA version 14.0 (StataCorp, United States).
InterventionThe intervention in each group is briefly reported here and in figure 1. The exercise program is described in detail in the section “Exercise protocol” in appendix 1.
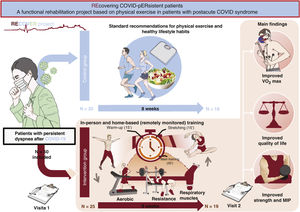
Central illustration. Patients with postacute COVID syndrome (PACS) with asthenia or dyspnea on exertion but without apparent changes in the cardiovascular or respiratory system were randomized to standard lifestyle and exercise recommendations (control group) or to a therapeutic physical exercise program (intervention group) for 8 weeks. The participants in the intervention group showed increased peak oxygen consumption (peak VO2) and improvements in quality of life scales, strength variables, respiratory muscles (maximal inspiratory pressure), and mitochondrial function parameters. No changes were seen in these variables in the control group. MIP, maximal inspiratory pressure.
IG patients completed an 8-week TPEP comprising an in-person modality and another modality that was conducted at home with remote monitoring (via heart rate monitors). The latter comprised extensive aerobic training blocks (increasing intensity and volume from 40% to 65% of the peak VO2 intensity and from 30 to 60minutes) and respiratory muscle training blocks (2 daily sessions, 6 days a week). The in-person training included 3 aspects:
- •
Velocity-based neuromuscular training. In this training program, individuals performed each repetition at the maximum possible velocity, as assessed using linear encoders. In each set, a target velocity loss of 20% was permitted, as well as of 15% until the end of the training session. The load used was progressively increased from 60% to 80% of the single repetition maximum.
- •
High-intensity interval training. This type of exercise was included in the second phase of the intervention program (weeks 4-8).
- •
Training program for the core muscles.
The CG received recommendations on physical exercise and healthy habits based on recommendations for the general population.13 After 8 weeks of training, all patients underwent an assessment similar to that of visit 1.
EndpointsThe primary endpoint was the change in peak VO2 in mL/kg/min and as a percentage of the predicted value. The secondary endpoints were: a) change in quality of life scores (PCFS, EQ-5D-5L, and PHQ-9); b) change in maximal inspiratory pressure; c) change in neuromuscular capacity evaluated using load-velocity profiles for squat and bench press exercises; d) change in body fat percentage; and e) changes in mitochondrial function parameters: maximal fat and carbohydrate oxidation and load-time at the mitochondrial exhaustion point.
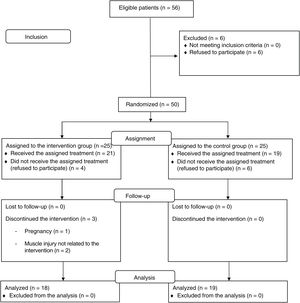
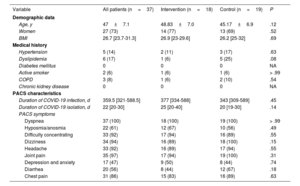
RESULTSBaseline characteristicsInitially, 56 patients with PACS were assessed; 50 were ultimately randomized (25 per group). Of these, 18 in the IG and 19 in the CG completed the protocol (73% women; mean age, 47 ± 7.1 years) (figure 2). The 2 groups exhibited balanced baseline characteristics.
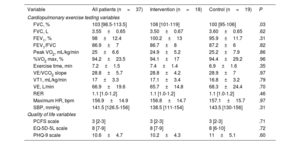
Table 2 shows the results for the variables obtained in the CPET, as well as those derived from the quality of life scales.
Baseline characteristics
| Variable | All patients (n=37) | Intervention (n=18) | Control (n=19) | P |
|---|---|---|---|---|
| Demographic data | ||||
| Age, y | 47±7.1 | 48.83±7.0 | 45.17±6.9 | .12 |
| Women | 27 (73) | 14 (77) | 13 (69) | .52 |
| BMI | 26.7 [23.7-31.3] | 26.9 [23-29.6] | 26.2 [25-32] | .69 |
| Medical history | ||||
| Hypertension | 5 (14) | 2 (11) | 3 (17) | .63 |
| Dyslipidemia | 6 (17) | 1 (6) | 5 (25) | .08 |
| Diabetes mellitus | 0 | 0 | 0 | NA |
| Active smoker | 2 (6) | 1 (6) | 1 (6) | > .99 |
| COPD | 3 (8) | 1 (6) | 2 (10) | .54 |
| Chronic kidney disease | 0 | 0 | 0 | NA |
| PACS characteristics | ||||
| Duration of COVID-19 infection, d | 359.5 [321-588.5] | 377 [334-588] | 343 [309-589] | .45 |
| Duration of COVID-19 isolation, d | 22 [20-30] | 25 [20-40] | 20 [19-30] | .14 |
| PACS symptoms | ||||
| Dyspnea | 37 (100) | 18 (100) | 19 (100) | > .99 |
| Hyposmia/anosmia | 22 (61) | 12 (67) | 10 (56) | .49 |
| Difficulty concentrating | 33 (92) | 17 (94) | 16 (89) | .55 |
| Dizziness | 34 (94) | 16 (89) | 18 (100) | .15 |
| Headache | 33 (92) | 16 (89) | 17 (94) | .55 |
| Joint pain | 35 (97) | 17 (94) | 19 (100) | .31 |
| Depression and anxiety | 17 (47) | 9 (50) | 8 (44) | .74 |
| Diarrhea | 20 (56) | 8 (44) | 12 (67) | .18 |
| Chest pain | 31 (86) | 15 (83) | 16 (89) | .63 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; NA, not applicable; PACS, postacute COVID syndrome.
Values represent No. (%), mean ± standard deviation, or median [interquartile range].
Quality of life and cardiopulmonary exercise testing data
| Variable | All patients (n=37) | Intervention (n=18) | Control (n=19) | P |
|---|---|---|---|---|
| Cardiopulmonary exercise testing variables | ||||
| FVC, % | 103 [98.5-113.5] | 108 [101-119] | 100 [95-106] | .03 |
| FVC, L | 3.55±0.65 | 3.50±0.67 | 3.60±0.65 | .62 |
| FEV1, % | 98±12.4 | 100.2±13 | 95.9±11.7 | .31 |
| FEV1/FVC | 86.9±7 | 86.7±8 | 87.2±6 | .82 |
| Peak VO2, mL/kg/min | 25±6.6 | 24.9±5.2 | 25.2±7.9 | .86 |
| %VO2 max, % | 94.2±23.5 | 94.1±17 | 94.4±29.2 | .96 |
| Exercise time, min | 7.2±1.5 | 7.4±1.4 | 6.9±1.6 | .35 |
| VE/VCO2 slope | 28.8±5.7 | 28.8±4.2 | 28.9±7 | .97 |
| VT1, mL/kg/min | 17±3.3 | 17.1±3.4 | 16.8±3.2 | .79 |
| VE, L/min | 66.9±19.6 | 65.7±14.8 | 68.3±24.4 | .70 |
| RER | 1.1 [1.0-1.2] | 1.1 [1.0-1.2] | 1.1 [1.0-1.2] | .46 |
| Maximum HR, bpm | 156.9±14.9 | 156.8±14.7 | 157.1±15.7 | .97 |
| SBP, mmHg | 141.5 [126.5-156] | 138.5 [111-154] | 143.5 [130-156] | .31 |
| Quality of life variables | ||||
| PCFS scale | 3 [2-3] | 3 [2-3] | 3 [2-3] | .71 |
| EQ-5D-5L scale | 8 [7-9] | 8 [7-9] | 8 [6-10] | .72 |
| PHQ-9 scale | 10.6±4.7 | 10.2±4.3 | 11±5.1 | .60 |
EQ-5D-5L, EuroQol scale; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HR, heart rate; PCFS, Post-COVID-19 Functional Status; peak VO2, maximal oxygen uptake during the test; PHQ-9, Patient Health Questionnaire-9; RER, respiratory exchange ratio; SBP, systolic blood pressure; %VO2 max, peak oxygen uptake as a percentage of the predicted maximal oxygen uptake; VCO2, carbon dioxide production; VE, minute ventilation; VE/VCO2 slope, ventilatory efficiency; VT1, first ventilatory threshold.
Values represent No. (%), mean ± standard deviation, or median [interquartile range].
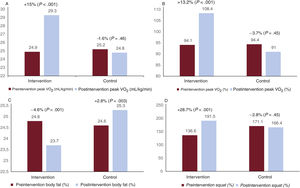
Peak VO2 significantly improved by 15% after the TPEP in the IG (peak VO2 pre- vs postintervention, 24.9% vs 29.3% mL/kg/min; P < .001). Predicted %VO2 max improved by 13.2% (%VO2 max pre- vs postintervention, 94.1% vs 108.4%; P < .001). In contrast, the peak VO2 in the CG showed no significant changes (peak VO2 pre- vs postintervention, 25.2 vs 24.8mL/kg/min; P=.46; predicted %VO2 max pre- vs postintervention, 94.4% vs 91%; P=.46) (figure 1 and figure 3).
Primary endpoint results (A and B) and some secondary endpoints (C, body fat percentage; D, change in the load-velocity curve for squat performance). The panels illustrate the change from visits 1 to 2 in the intervention and control groups. Panel A shows the change in peak oxygen consumption (VO2). Panel B shows the changes over time in VO2 as a percentage of the predicted VO2 max for the population according to Wasserman/Hansen equations.
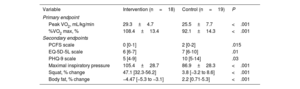
The functional improvement reflected in the VO2 was correlated with improvements in quality of life scales (table 3). Other secondary endpoints, such as maximal inspiratory pressure, neuromuscular capacity (squat and bench press), and body fat percentage, improved after the intervention in the IG vs the CG (table 4).
Endpoints
| Variable | Intervention (n=18) | Control (n=19) | P |
|---|---|---|---|
| Primary endpoint | |||
| Peak VO2, mL/kg/min | 29.3±4.7 | 25.5±7.7 | <.001 |
| %VO2 max, % | 108.4±13.4 | 92.1±14.3 | <.001 |
| Secondary endpoints | |||
| PCFS scale | 0 [0-1] | 2 [0-2] | .015 |
| EQ-5D-5L scale | 6 [6-7] | 7 [6-10] | .01 |
| PHQ-9 scale | 5 [4-9] | 10 [5-14] | .03 |
| Maximal inspiratory pressure | 105.4±28.7 | 86.9±28.3 | <.001 |
| Squat, % change | 47.1 [32.3-56.2] | 3.8 [−3.2 to 8.6] | <.001 |
| Body fat, % change | −4.47 [−5.3 to −3.1] | 2.2 [0.71-5.3] | <.001 |
EQ-5D-5L, EuroQol scale; PCFS, Post-COVID-19 Functional Status; PHQ-9, Patient Health Questionnaire-9; VO2, oxygen uptake.
Values represent No. (%), mean ± standard deviation, or median [interquartile range].
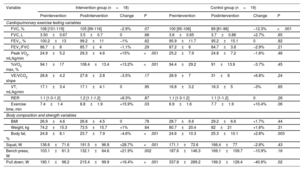
Change in cardiopulmonary exercise testing, body composition, and strength variables
| Variable | Intervention group (n=18) | Control group (n=19) | ||||||
|---|---|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Change | P | Preintervention | Postintervention | Change | P | |
| Cardiopulmonary exercise testing variables | ||||||||
| FVC, % | 108 [101-119] | 105 [99-116] | −2.9% | .07 | 100 [95-106] | 89 [81-96] | −12.3% | <.001 |
| FVC, L | 3.50±0.67 | 3.5±0.7 | 0 | .68 | 3.6±0.65 | 3.7±0.88 | +2.7% | .65 |
| FEV1, % | 100.2±13 | 99.2±11 | −1% | .62 | 95.9±11.7 | 95.2±15.1 | 0 | .82 |
| FEV1/FVC | 86.7±8 | 85.7±4 | −1.1% | .29 | 87.2±6 | 84.7±3.8 | −2.9% | .21 |
| Peak VO2, mL/kg/min | 24.9±5.2 | 29.3±4.6 | +15% | <.001 | 25.2±7.9 | 24.8±7.2 | −1.6% | .46 |
| %VO2 max, % | 94.1±17 | 108.4±13.4 | +13.2% | <.001 | 94.4±29.2 | 91±13.9 | −3.7% | .45 |
| VE/VCO2 slope | 28.8±4.2 | 27.8±2.8 | −3.5% | .17 | 28.9±7 | 31±8 | +6.8% | .24 |
| VT, mL/kg/min | 17.1±3.4 | 17.1±4.1 | 0 | .95 | 16.8±3.2 | 16.3±5 | −3% | .65 |
| RER | 1.1 [1.0-1.2] | 1.2 [1.1-1.2] | +8.3% | .87 | 1.1 [1.0-1.2] | 1.1 [1-1.2] | 0 | .26 |
| Exercise time, min | 7.4±1.4 | 8.8±1.9 | +15.9% | .03 | 6.9±1.6 | 7.7±1.9 | +10.4% | .06 |
| Body composition and strength variables | ||||||||
| BMI | 26.9±4.6 | 26.8±4.5 | 0 | .78 | 28.7±6.6 | 29.2±6.6 | +1.7% | .44 |
| Weight, kg | 74.2±15.3 | 73.5±15.7 | +1% | .64 | 80.7±20.4 | 82±21 | +1.6% | .31 |
| Body fat, % | 24.8±8.1 | 23.7±7.9 | −4.6% | <.001 | 24.6±10.3 | 25.3±10.1 | +2.8% | .003 |
| Squat, W | 136.6±71.6 | 191.5±96.8 | +28.7% | <.001 | 171.1±72.6 | 166.4±77 | −2.8% | .43 |
| Bench press, W | 103.1±61.3 | 132.1±64.6 | +21.9% | .002 | 187.6±146.3 | 169.1±109.7 | −10.9% | .16 |
| Pull down, W | 180.1±98.2 | 215.4±99.9 | +16.4% | <.001 | 337.8±289.2 | 199.3±128.4 | −40.9% | .02 |
BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; peak VO2, maximal oxygen uptake during the test; RER, respiratory exchange ratio; %VO2 max, peak oxygen uptake as a percentage of predicted maximal oxygen uptake; VCO2, carbon dioxide production; VE, minute ventilation; VE/VCO2 slope, ventilatory efficiency.
Values represent No. (%), mean ± standard deviation, or median [interquartile range].
The IG reached the mitochondrial exhaustion point at higher loads and a higher heart rate (P < .001) without significant changes in mitochondrial fat oxidation (FATox) (table 1 of the supplementary data). This indicates longer use of fat as substrate during exercise and an increase in the area under the curve for FATox, demonstrating better energy efficiency. The maximal carbohydrate oxidation also increased (P < .001). Meanwhile, both parameters were unchanged in the CG. These results indicate improved metabolic flexibility in the IG (table 1).
DISCUSSIONThe RECOVER study is the first randomized clinical trial to use CPET to assess the impact of a TPEP in patients with PACS and EI. The most pertinent findings are the following: a) the TPEP improved functional capacity, as evaluated using peak VO2, in patients with PACS and EI; b) this improvement was associated with enhanced quality of life; c) the TPEP improved strength and body composition (fat percentage); and d) the TPEP was related to improvements in metabolic flexibility and mitochondrial function indirectly estimated using CPET.
Our results revealed improvements of 15% in the peak VO2 and of 13.2% in the predicted VO2max in the IG and no significant changes in the CG. This improvement in functional capacity might underlie the improvements in the quality of life scores.
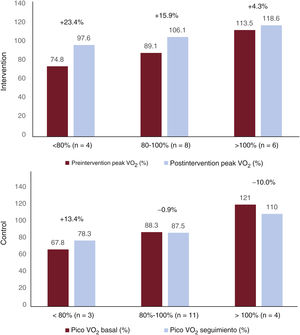
The baseline VO2 was 94%, similar to that reported for mild and moderate cases after a 1-year clinical course.11,14–17 This VO2, exceeding 80%, does not rule out the presence of a significant clinical limitation. Differences in the predicted VO2 have been reported between symptomatic and asymptomatic patients (92% vs 107.3%), which indicates the relationship between PACS symptoms and the VO2.17 In addition, the symptom improvement was related to an improved peak VO2, which reveals an amelioration of the physical condition deterioration that is probably related to the virus.18 However, the peak VO2 often remains reduced 12 months after the infection.17 The baseline VO2 of our sample, close to normal, strengthens the effectiveness of the TPEP because, in line with the Wilder principle, it is more difficult to find significant differences when the initial values are higher.19 In addition, sample stratification by baseline VO2 (figure 4) revealed a gradient of improvement, with the participants with the lowest baseline VO2 improving the most.
Change in peak oxygen consumption (VO2) during follow-up based on baseline peak VO2. The figure shows the pre- and postintervention change in VO2 in the control and intervention groups (in bold, the percentage difference). There is a gradient of improvement according to baseline VO2 in the intervention group, with patients with a lower baseline VO2 obtaining greater benefit from the therapeutic physical exercise program, although the improvement was evident in the entire intervention group.
In agreement with other studies,20,21 symptoms of anxiety-depression were seen and the PHQ-9 score was 12.1 (indicating moderate depression). Functional status was quantified using the PCFS scale, which is specific for PACS22 and which is scored from 0 (no limitation) to 4 (severe functional limitation). Both groups had a mild-to-moderate limitation at baseline. TPEP was associated with a decrease in anxiety-depression in the IG while no change was seen in the CG. Regarding functional status, TPEP led to a significant improvement in the PCFS scale, specifically in the IG, probably due to the effect of exercise on neuroplasticity, by reducing the allostatic load induced by the convalescent period.23
Peripheral neuromuscular involvement is one of the main hypotheses concerning the origin of the EI in patients with PACS.9,24 Various theories have been advanced to explain the pathogenesis of the peripheral dysfunction, and our group proposes that metabolic-mitochondrial dysfunction is the main driver of the EI, based on the current evidence and the exploratory findings reported here. Patients with PACS have mitochondrial dysfunction, indirectly demonstrated using the FATox rate and lactate use (a method described and validated by San Millán and Brooks25) and, recently, metabolomic studies.26 This mitochondrial dysfunction is even present when patients with PACS are compared with individuals with metabolic syndrome, who show a lower rate of FATox and a greater accumulation of lactate at lower exercise loads.24 CD147, a mitochondrial transmembrane glycoprotein from the immunoglobulin superfamily, has been suggested to serve as a gateway for SARS-CoV-2 by acting as a receptor in host cells for the viral spike protein.27 In addition, CD147 is implicated in the mitochondrial surface expression of the monocarboxylate transporters MCT1 and MCT4, responsible for transporting cytosolic lactate to the mitochondrial interior or to adjacent cells for its subsequent use as an energy source. Blockade or saturation of CD147 by the virus could lead to lower expression of the MCT1 and -4 transporters, which would inevitably culminate in the inability of mitochondria to internalize and use the lactate produced.28 Accordingly, the poor clearance of lactate from the cells, in conjunction with the lower FATox capacity, would result in mitochondrial dysfunction, the cause of the EI symptoms and the functional incapacity.
Physical exercise exerts beneficial effects on mitochondrial dynamics, by stimulating, first, mitochondrial fusion, mitophagy, and mitochondrial biogenesis, and, second, the transformation of fast-twitch muscle fibers (type IIa), which are largely used for glycolytic metabolism, into slow-twitch fibers (type I), which have higher oxidative capacity for fatty acids. In our study, although no data were available on lactate levels, we used CHOox (carbohydrate oxidation) as an indirect measure of glycolytic function, which overall indicated higher metabolic flexibility after the intervention. Although maximal FATox was not significantly changed, the improvement in metabolic flexibility was related to a more efficient use of fat during exercise, via an improved time and load at which the mitochondrial exhaustion point was reached (with an increased area under the curve of FATox during exercise). The preferential use of lipids allows carbohydrates to be reserved for higher intensity exercise, which indicates greater energy efficiency.
A novel aspect of the training method is the clinical use of a resistance training method,29 used in the field of physical performance. In the physical performance field, a paradigm shift has occurred in the last 2 decades, from load-based resistance training (LRT), based on a voluntary maximum number of repetitions, to velocity-based resistance training (VRT) methods. Linear encoders are used in the latter approach because they allow real-time measurement of the velocity of each repetition. This method is based on individuals performing each repetition at the maximum velocity possible. The number of repetitions is not predetermined but is adjusted to the percentage of velocity loss, that is, the degree of fatigue experienced by the individual. This permits individualization of the load of each session based on the degree of strength improvement (allowing greater loads) and of the accumulated fatigue (leading to reduced loads). However, in LRT, the reference is an estimated maximum repetition or a specific measurement at training program initiation; this weight is fixed and invariable during the program. It was first reported at the end of the 1990s that these exercises had to be performed at a specific velocity to improve strength, meaning that adequate adherence to VRT achieves better strength improvements than LRT.29 Beyond their greater effectiveness, one of the most relevant aspects of their implementation is the possible individualization and assessment of fatigue in VRT, which improves safety and avoids overtraining. Both are extremely important not only for strength improvements, but also for achieving adherence (a vital aspect in this clinical context).
No significant changes were detected in the first ventilatory threshold after the intervention. This was partly due to the design of the TPEP, which was focused on improving the primary endpoint (peak VO2) and not particularly on zone 2 training, the type of exercise that more directly affects this threshold. In addition, the first ventilatory threshold was within the normal range, a frequent finding in PACS, particularly in individuals who did not require hospitalization,30,31 which hinders the achievement of pertinent changes.
As well as resistance training, inspiratory muscle training significantly ameliorates symptoms in patients with PACS.32 In our study, the TPEP included training of the respiratory muscles, and the results revealed a significant improvement in the maximal inspiratory pressure of the patients in the IG, which boosted their functional and symptomatic recovery.
In addition to the above-mentioned possible causes of EI in PACS, the complex and as-yet-unknown pathophysiology of PACS may include autonomic dysfunction, disproportionate hyperventilation for the degree of exercise, chronotropic incompetence, and immune system dysregulation.33–36 It remains to be elucidated whether the EI involves these entities or other pathophysiological mechanisms, as well as whether it actually comprises a COVID-19-specific mechanism or one common to a variety of diseases. Understanding the pathogenesis of EI in patients with PACS would enable the identification of appropriate treatments but structured TPEPs may currently be the best treatment, given the promising results from our study.
Due to the prevalence of PACS, it is impossible to offer TPEPs in specific units to all patients. Cardiac rehabilitation units, given their experience with the prescription of TPEPs, should play a starring role in the treatment of PACS. For patients without access to these units, the challenge will be to develop a standardized home-based TPEP that includes polarized aerobic training (low and high intensity), resistance training (without the need for specific machines), and respiratory training, so that all patients can benefit from our findings. The impact of the absence of certain equipment or a personal trainer will be minimized by extending the program beyond the 8-week duration of our intervention.
LimitationsThis study has several limitations. Although sufficiently powered to find statistically significant differences, the sample size is small. Second, there is volunteer bias, inherent to this type of clinical trial. Finally, mitochondrial function parameters were added as a secondary endpoint after study initiation.
CONCLUSIONSTPEPs in patients with PACS and EI improve functional capacity evaluated using VO2. This is accompanied by improvements in perceived quality of life, strength, body fat percentage, and metabolic-mitochondrial function parameters (metabolic flexibility).
FUNDINGUnconditional grant from Fundación Soliss.
AUTHORS’ CONTRIBUTIONSThe manuscript authors accept full responsibility for the manuscript content, as defined by the International Committee of Medical Journal Editors. J. Godoy López and M. Gallango Brejano were in charge of the design and application of the training program. A. Berenguel Senén, A. Gadella Fernández, J. Borrego Rodríguez, P. Cepas Guillén, J. Godoy López, and M. Gallango Brejano participated in the manuscript writing and revision. The remaining authors contributed to the design or development of the study.
- –
PACS is a frequent condition after acute COVID-19 infection whose most common symptom is exercise intolerance. The literature shows the presence of peripheral neuromuscular involvement.
- –
The RECOVER study is the first randomized clinical trial to use CPET to assess the role of a TPEP in patients with PACS. The results show that the TPEP effectively improved functional capacity (measured using VO2 change), quality of life, strength, mitochondrial function parameters, and body composition.
The authors declare no conflicts of interest.