Different studies have shown improvement in patients with idiopathic nonischemic dilated cardiomyopathy treated with cell-therapy. However, factors influencing responsiveness are not well known. This trial investigates functional changes and factors influencing the 6-month gain in ejection fraction in 27 patients with dilated cardiomiopathy treated with intracoronary cell-therapy.
MethodsPatients received intracoronary infusion of autologous bone-marrow mononuclear cells (mean infused, 10.2 [2.9]×108). Flow cytometry and functional analyses of the cells were also performed.
ResultsThe 6-month angiographic gain in ejection fraction ranged from −9% to 34% (mean, 9%). These changes were distinguished into 2 groups: 21 patients (78%) with a significant improvement at the 6-month evaluation (mean gain, 14 [7]%), and 6 patients who had no response (mean gain, −5 [3]%). The responders were younger as compared to the nonresponders (50 [12] years vs 62 [9] years; P<.04). There was an inverse correlation (r=−0.41; P<.003) between the gain in ejection fraction and the high density lipoprotein level, suggesting higher functional gain with low high density lipoprotein levels. The 24h migratory capability of the infused cells was significantly reduced in the responders’ group (5.4 [1.7]×108 vs 8.1 [2.3]×108; P<.009 for vascular endothelial growth factor and 5.8 [1.7]×108 vs 8.4 [2.9]×108; P<.002 for stromal cell-derived factor-1).
ConclusionsYounger patients with dilated cardiomiopathy and lower plasma high density lipoprotein levels gain greater benefit from intracoronary cell-therapy. Functional improvement also seems to be enhanced by a lower migratory capacity of the infused cells.
Keywords
.
INTRODUCTIONIntracoronary infusion of autologous bone marrow-derived mononuclear cells (BMMNC) has been used for functional recovery in patients with acute1–3 and chronic4,5 ischemic heart disease. However, less information is available on the effects of BMMNC in patients with nonischemic dilated cardiomyopathy (DC). Different studies have shown clinical improvement and a mild benefit in left ventricular function in patients with idiopathic DC treated with cell-therapy.6–12 However, the degree of functional improvement varies among patients, and little is known about the factors influencing such a response. This trial investigates the factors influencing the 6-month gain in left ventricular ejection fraction (LVEF) in 27 patients with DC included in a trial on intracoronary cell-therapy.
METHODSStudy ProtocolPatients with idiopathic DC were proposed to participate in the trial. The study protocol (TCMR0007/06) was approved by all appropriate institutional, regional, and national committees. The trial was presented as a noncontrolled phase II clinical trial (EudraCT: 2007-003088-36) and was registered on www.clinical-trials.gov (NCT00629096). The main objective of the trial was to evaluate the safety and efficacy of intracoronary infusion of BMMNC in patients with idiopathic DC who would be followed-up for 1 year. A biological analysis was designed to relate the marrow cell composition and function to possible changes in clinical and functional evolutions. The inclusion criteria were as follows: a) patients with a clinical and angiographic diagnosis of idiopathic DC, with an LVEF<50% and signs and symptoms of heart failure, despite optimally personalized medical treatment, and b) a stable sinus rhythm. The exclusion criteria were as follows: a) DC of a known origin (toxic, ischemic, secondary to chemotherapy, or deposits); b) a recent history of myocarditis; c) patients with criteria of asynchrony who might benefit from resynchronization therapy; d) patients on the waiting list for cardiac transplantation; e) patients older than 80 years or having other systemic or hemathological disorders; f) pregnant women or women likely to become pregnant, and g) patients enrolled in any other type of clinical trial.
All patients, after providing written informed consent, were studied clinically and functionally at 4 stages: baseline (before treatment) and at 3, 6, and 12 months after the cell infusion. A serial echocardiographic evaluation was performed before the treatment, at 3 months, and at 1 year. An angiographic study was performed before the treatment and at the 6-month evaluation. Changes in left ventricular function were analyzed and considered to be the primary end point. The net gain in the LVEF (angiographic) was considered to be the main functional variable. As secondary end points, we evaluated the clinical course, coronary flow reserve, and echocardiographic follow-ups, together with the biological properties of the infused cells.
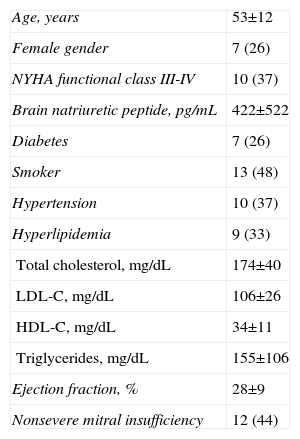
PatientsPatients were enrolled in the trial from March 2008 to December 2010. There were no patients who entered the trial after a short evolution process since diagnosis. All patients had a chronic stable evolution before entering the trial. The age at treatment ranged from 31 years to 74 years (mean, 53 [12]). Twenty patients were male and 7 patients were female. Three patients had required an automatic defibrillator to prevent lethal arrhythmias. Table 1 shows the main baseline clinical data. On entry, all patients were stable and under individualized medical treatment according to their clinical conditions and New York Heart Association (NYHA) functional classes. All patients received angiotensin-converting-enzyme inhibitors or angiotensin-II-receptor antagonist, loop diuretics, β-blockers, and spironolactone or eplerenone. Seven patients (26%) had medically treated diabetes. Eight patients (30%) were on acenocumarol and 19 patients (70%) were on aspirin. Twelve patients (44%) were on digoxin, and 9 patients (33%) with hyperlipidemia were taking statins. The medical treatment remained stable along the trial in most patients (n=20). In 1 patient, with 2 episodes of heart failure worsening, the medical treatment was increased. In 6 patients the number of drugs was reduced when a clear functional class improvement at follow-up made it possible. Thus, the pharmacologic treatment was mostly unchanged throughout the trial and was even mildly decreased in a few patients.
Main Baseline Clinical Data.
| Age, years | 53±12 |
| Female gender | 7 (26) |
| NYHA functional class III-IV | 10 (37) |
| Brain natriuretic peptide, pg/mL | 422±522 |
| Diabetes | 7 (26) |
| Smoker | 13 (48) |
| Hypertension | 10 (37) |
| Hyperlipidemia | 9 (33) |
| Total cholesterol, mg/dL | 174±40 |
| LDL-C, mg/dL | 106±26 |
| HDL-C, mg/dL | 34±11 |
| Triglycerides, mg/dL | 155±106 |
| Ejection fraction, % | 28±9 |
| Nonsevere mitral insufficiency | 12 (44) |
HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; NYHA, New York Heart Association.
Data are expressed as no. (%) or mean±standard deviation.
The clinical evaluation was based on the NYHA functional class, and was clearly established at every stage of the trial. In addition, exercise capacity, as evaluated by the peak oxygen uptake in mL/kg/min, was determined before treatment and at 3 months and 1 year after the treatment. The exercise testing was performed with a Marquette Case 8000 system (GE Healthcare, Burr Ridge, Illinois, United States) using a Naughton protocol in accordance with the guidelines.13 The respiratory gas exchange during maximal effort was obtained with an ergoespirometry testing station (Oxycon Pro/Delta, Viasys Healthcare; Hochberg, Germany). The peak oxygen uptake measurements were obtained and expressed in both, absolute values and as a percentage of the theoretical values estimated for age and body surface area. The exercise time was also recorded and considered to further be a functional parameter.
Echocardiograhic StudiesAt all 3 stages (baseline, 3 months, and 1 year), we performed a complete echocardiographic study (Philips iE33, Philips Ultrasound, Philips Healthcare; Massachusetts, United States) with second harmonic imaging. The left ventricular volumes and LVEF were obtained using the biplanar Simpson's rule. In all instances, echo-enhancers were intravenously injected to optimize the definition of the endocardial borders. Mitral insufficiency, when present, was studied by continuous and color Doppler, and graded in severity from I to IV. To define each grade, mitral regurgitation was assessed integrally with agreement by 2 expert echocardiographers, following the recommendations of the American Society of Echocardiography.14
Hemodynamic and Angiographic StudiesBefore treatment and 6 months after cell infusion, cardiac catheterization was performed to obtain the systemic and left ventricular end-diastolic pressure, to study the left ventricular function, and to analyze the coronary flow reserve for each coronary artery. The left ventricular function studies were based on the analysis of at least 1 left ventricular angiogram at a 30o right anterior oblique projection. During each ventriculogram, attempts were made to obtain a sinus beat and a post-extrasystolic beat for analysis to ascertain the contractile reserve of the ventricle.
The end-diastolic and end-systolic silhouettes were drawn with the CASS system by consensus of 2 expert angiographers. The left ventricular volumes and LVEF were derived and the regional wall motion was analyzed. Sheehan's method15 was used for the asynergy study, dividing the superimposed silhouettes into 100 radii of wall shortening, from end-diastole to end-systole. The abnormal contracting segment was defined as the percentage of radii showing akinesis or diskinesis. The competence of the mitral valve was evaluated and mitral insufficiency, when present, was graded in severity from I to IV.
The measurement of the velocities and coronary flow reserve in each coronary artery was also performed in both stages using the FloMap® system (Cardiometrics; Mountain View, California, United States). A 0.014-inch intracoronary Doppler guide wire was positioned in the proximal segments of all 3 coronary arteries and the flow velocity was recorded continuously. The averaged peak velocity was obtained at baseline and after an intracoronary bolus of 60μg of adenosine for the left coronary artery and 40μg of adenosine for the right coronary artery. The coronary flow reserve was calculated as the ratio between the maximal flow velocity during the peak effect of the adenosine injection and the baseline flow velocity.
Cell Harvesting, Suspension Preparation, and Intracoronary InfusionA volume of marrow (100mL to 150mL) was harvested under local anaesthesia. The BMMNCs were isolated by density gradient centrifugation over Ficoll-Hypaque solution in a sterile sepax device. After 3 washes, the BMMNCs were filtered and resuspended in 10mL of 0.9% sodium chloride supplemented with 0.1% heparin. The preparation of the cell suspension lasted no more than 4h and was then transported to the lab for cell infusion in synchrony with duly completed diagnostic cardiac catheterization. Based on the coronary anatomy, a decision was made to divide the cell suspension according to the dominant artery. A total of 10.2 (2.9)×108 BMMNCs were infused into the coronary arteries as follows: 50% of the cell suspension was infused into the left anterior descending artery and 50% was infused into the right coronary artery in 4 patients (15%); 50% of the cell suspension was infused into the left anterior descending artery and 50% was infused into a dominant circumflex artery in 6 patients (22%); 50% of the cell suspension was infused into the left anterior descending artery, 25% was infused into the right coronary artery, and the remaining 25% was infused into the circumflex artery in 16 patients (59%); and the cell suspension was distributed equally (33% each) in all 3 coronary arteries in 1 patient (4%) who had a small left anterior descending artery. Selective infusion in every artery was performed through a micro-catheter, which was subsequently placed in a proximal segment of each coronary artery. Every intracoronary infusion lasted 2min to 4min, without conditions of stagnant flow. After infusion, the patients were transferred to the floor for continuous 24h monitoring and serial enzymatic testing.
Biological StudyAliquots of the final BMMNC product were obtained for automated cell counting, flow cytometry, functional analysis of colony-forming unit endothelial cells, and their migratory capacity. The cells were labelled with monoclonal antibodies linked to fluorescein isothiocyanate or allophycocyanin against human CD34 (BD Pharmingen™; San Diego, California, United States) and monoclonal antibodies linked with fluorescein isothiocyanate or phycoerythrin against the following human cell-surface proteins: CD38, CXCR4, CD31, CD146, vascular endothelial growth factor R2 (VEGFR2) (BD Pharmingen™), CD90 (R&D Systems; Minneapolis, Minnesota, United States), HLA-DR, and CD117 (BD Biosciences; San Jose, California, United States), or a monoclonal antibody coupled to allophycocyanin, antihuman CD133 (Miltenyi Biotec; Bergisch Gladbach, United Kingdom). The cell samples were acquired on a FACSCanto™ flow cytometer from Beckton and Dickinson (BD Biosciences) with dual laser, and were analyzed using the BD CellQuest Pro™ software (BD Biosciences). At least 2000 events were analyzed for each marker.
The functional analysis of the migratory capability of the infused cells was performed in a membrane insert with polyethylene terephtalate, with a 6.5mm diameter and with 8μm pores (BD Falcon™ Cell Culture Insert System; Franklin Lakes, New Jersey, United States) as follows: a) the insert was placed in a culture dish of a 24-well plate (BD Falcon™); b) the lower chamber of the insert was filled with RPMI-1640 (BioWhittaker® Lonza; Verviers, Belgium) plus stromal cell-derived growth factor-1α (SDF-1α; 100 ng/mL; R&D Systems), or RPMI-1640 plus vascular endothelial growth factor (VEGF 165; 100 ng/mL; R&D Systems); or RPMI alone. The upper chamber of the insert was loaded with 5×10 mononuclear cells resuspended in RPMI-1640 serum-free medium. The plates were incubated at 37°C with 95% humidity and 5% CO2 for 24h and thereafter, observed under an inverted microscope with automatic counting system fields that allowed the calculation of the percentage of migrated cells.
Statistical StudyThe data are expressed as means (standard deviation). Differences between the proportions were studied using the chi-square test or Fisher's exact test, as appropriate. Nonparametric tests were used (Wilcoxon test for paired data and Mann-Whitney U test for independent data) to compare mean values. The differences between the 3 stages of the noninvasive parameters in the same patient were analyzed using a nonparametric test for k related samples (Friedman test). The linear correlation between quantitative variables was analyzed using the Pearson test. Values of P<.05 were considered to be statistically significant.
RESULTSCell Suspension CharacteristicsThe numbers of infused cells were as follows: BMMNC (×108), 10.2 (2.9); CD34+ (×106), 40.4 (23.6); CD34+/CD117+ (×106), 20.6 (15.4); CD133+ (×106): 22.8 (15.3); CD34+/CD38- (×106), 9.9 (11.8); CD34+/CXCR4+ (×106), 1.9 (2.8), and CD90+ (×106), 1.3 (1.3). The total number of red blood cells contaminating the suspension was 0.49 (0.23) (×108), with a hematocrit value of 0.42% (0.2)%. Regarding the main functional characteristics, the migration to VEGF (×108) was 5.8 (2) cells, the migration to SDF-1 (×108) was 6.3 (2.1) cells, and the frequency of colony-forming unit endothelial cells (×106) was 8.2 (13.9) cells.
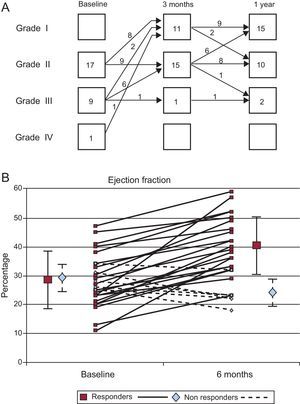
Clinical FindingsTable 1 shows the main baseline clinical data. There were no major complications related to the intracoronary cell transfer or any other procedure during the study protocol. During the infusion of the cell suspension in the right coronary artery, mild bradycardia and hypotension were observed in 5 patients; intravenous fluids and slowing the rate of infusion allowed the procedure to be completed without complications. No arrhythmias or myocardial damage was recorded during the following 24h (mean peak troponin I was 0.03 [0.04] ng/mL). During a close follow-up for 1 year, only 1 patient needed rehospitalization twice due to worsening heart failure. The remaining 26 patients continued to evolve to a better functional class. Figure 1A shows the course of the NYHA functional class in our patients. Only 2 patients, out of 10, remained in functional class III after 1 year. This coincided with a significant reduction in the circulating N-terminal pro-brain natriuretic peptide (NT-proBNP) levels throughout the trial: baseline, 403 (417) pg/mL; 3-month, 129 (169) pg/mL; and 1-year, 122 (190) pg/mL (P<.05).
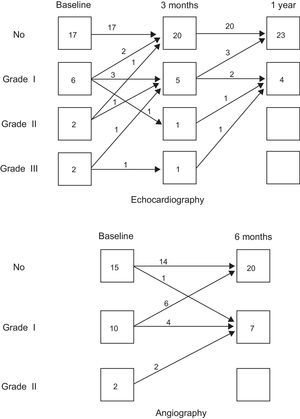
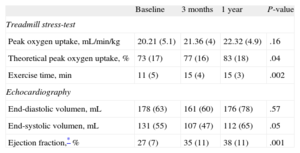
Noninvasive Functional FindingsTable 2 shows the course observed in the noninvasive parameters. The exercise capacity improved in most patients. The theoretical peak oxygen uptake and the exercise time improved serially. There were also significant improvements in the echocardiographic left ventricular volumes and LVEF. In addition, there was a frequent reduction in the degree of mitral regurgitation, when present, as evaluated by echo-Doppler analysis. Figure 2A shows the changes observed in the degree of mitral insufficiency throughout the echocardiographic study.
Evolution of Noninvasive Parameters.
| Baseline | 3 months | 1 year | P-value | |
| Treadmill stress-test | ||||
| Peak oxygen uptake, mL/min/kg | 20.21 (5.1) | 21.36 (4) | 22.32 (4.9) | .16 |
| Theoretical peak oxygen uptake, % | 73 (17) | 77 (16) | 83 (18) | .04 |
| Exercise time, min | 11 (5) | 15 (4) | 15 (3) | .002 |
| Echocardiography | ||||
| End-diastolic volumen, mL | 178 (63) | 161 (60) | 176 (78) | .57 |
| End-systolic volumen, mL | 131 (55) | 107 (47) | 112 (65) | .05 |
| Ejection fraction,* % | 27 (7) | 35 (11) | 38 (11) | .001 |
Data are expressed as mean (standard deviation).
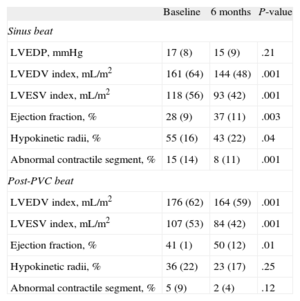
Table 3 shows the observed evolution of the main hemodynamic and angiographic parameters. No changes were observed in the Doppler peak flow velocity and the coronary flow reserve of all 3 coronary arteries at the 6-month follow-up. The mean coronary flow reserve was normal in all 3 arteries at baseline study (right coronary artery, 2.5 [0.8]; left anterior descending artery, 2.6 [0.8], and circumflex artery, 2.3 [0.6]). Six months after treatment the coronary flow reserve of all 3 coronaries remained unchanged (2.8 [0.8], 2.5 (0.4), and 2.7 [1], respectively). Regarding left ventricular function, there were significant reductions in left ventricular volumes and a gain in LVEF that coincided with a reduction in abnormal regional wall shortening (mean reduction of hypokinetic radii: 12.9% [20]%) and a reduction of akinetic segments, when present at the baseline study (mean reduction of abnormal contracting segment: 7.1% [9.4]%). The contractile reserve of the ventricle, as evaluated by postextrasystolic potentiation, improved proportionally 6 months after cell-therapy. Twelve patients had some degree of angiographic mitral insufficiency before treatment. Six months after cell-therapy, 8 of the patients showed a reduction in the degree of angiographic mitral regurgitation and 1 patient showed an increase of 1 degree (Fig. 2).
Left Ventricular Functional Changes (Hemodynamic and Angiographic).
| Baseline | 6 months | P-value | |
| Sinus beat | |||
| LVEDP, mmHg | 17 (8) | 15 (9) | .21 |
| LVEDV index, mL/m2 | 161 (64) | 144 (48) | .001 |
| LVESV index, mL/m2 | 118 (56) | 93 (42) | .001 |
| Ejection fraction, % | 28 (9) | 37 (11) | .003 |
| Hypokinetic radii, % | 55 (16) | 43 (22) | .04 |
| Abnormal contractile segment, % | 15 (14) | 8 (11) | .001 |
| Post-PVC beat | |||
| LVEDV index, mL/m2 | 176 (62) | 164 (59) | .001 |
| LVESV index, mL/m2 | 107 (53) | 84 (42) | .001 |
| Ejection fraction, % | 41 (1) | 50 (12) | .01 |
| Hypokinetic radii, % | 36 (22) | 23 (17) | .25 |
| Abnormal contractile segment, % | 5 (9) | 2 (4) | .12 |
LVEDP, left ventricular end-diastolic pressure; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; PVC, premature ventricular contraction.
Data are expressed as mean (standard deviation).
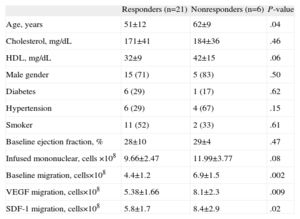
The gain in LVEF was significantly higher in patients with lower LVEF (<25%) at baseline (16% [8%] vs 6% [9%]; P<.01). The individual changes in LVEF are shown in Figure 1B. There was a clear distinction between the 2 groups of patients: 21 patients (78%) with a significant improvement at the 6-month evaluation (mean gain, 14% [7%]) vs 6 patients with no response or even worsening (mean gain −5% [3%]) at the 6-month evaluation. Although the second group was small, we analyzed possible factors associated with responsiveness. Table 4 shows the univariate analysis. The responders were younger. Interestingly, the 24h migratory capacity of the cell suspension was significantly lower in these responders.
Factors Influencing Responsiveness (Univariate Analysis).
| Responders (n=21) | Nonresponders (n=6) | P-value | |
| Age, years | 51±12 | 62±9 | .04 |
| Cholesterol, mg/dL | 171±41 | 184±36 | .46 |
| HDL, mg/dL | 32±9 | 42±15 | .06 |
| Male gender | 15 (71) | 5 (83) | .50 |
| Diabetes | 6 (29) | 1 (17) | .62 |
| Hypertension | 6 (29) | 4 (67) | .15 |
| Smoker | 11 (52) | 2 (33) | .61 |
| Baseline ejection fraction, % | 28±10 | 29±4 | .47 |
| Infused mononuclear, cells ×108 | 9.66±2.47 | 11.99±3.77 | .08 |
| Baseline migration, cells×108 | 4.4±1.2 | 6.9±1.5 | .002 |
| VEGF migration, cells×108 | 5.38±1.66 | 8.1±2.3 | .009 |
| SDF-1 migration, cells×108 | 5.8±1.7 | 8.4±2.9 | .02 |
HDL, high density lipoprotein; SDF-1, stromal cell-derived factor-1; VEGF, vascular endothelial growth factor.
Data are expressed as no. (%) or mean±standard deviation.
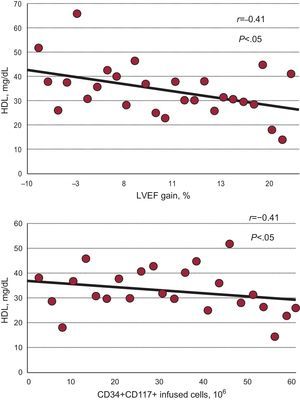
Additionally, the gain in LVEF inversely correlated to the baseline high density lipoprotein (HDL) (r=−0.41; P=.03), suggesting a higher gain in LVEF with lower HDL levels. In fact, HDL was significantly lower in those patients with a higher gain (>10%) in LVEF (30 [8] mg/dL vs 40 [10] mg/dL; P<.01). The baseline HDL also correlated inversely to the number of infused CD34+ CD 117+ cells (r=−0.41; P<.05) (Fig. 3).
DISCUSSIONDifferent experimental studies in rabbit and rat models of DC have shown a functional benefit after cell-therapy.16–20 Either using bone marrow aspirates of isogenic rats,16 cultured autologous skeletal myoblasts,17 or cultured mesenchymal cells18–20, the cell suspension was directly injected into the myocardium. Based on these experimental findings, different clinical studies6–12 have analyzed the safety and efficacy of different types of cell-therapy in patients with nonischemic DC. In the year 2006, Seth et al.6 first reported an improvement in the NYHA functional class, a decrease in the left ventricular end-systolic volume, and a 5.4% increase in LVEF in 24 patients with DC treated with intracoronary infusion of autologous BMMNC. In a late follow-up analysis of this trial,10 the functional benefits were sustained without any long-term side effects. Also, using intracoronary infusion of autologous BMMNC, Fisher-Rasokat et al.9 observed a 3-month improvement in the global and regional function, a trend in coronary flow reserve, and a significant decrease in NT-proBNP serum levels 1 year after treatment in 33 patients. In other studies,8,12 both BMMNC and peripheral blood stem cells obtained by apheresis, cultured or not, were surgically injected in the free wall of the left ventricle. These studies showed improvements in functional class or the 6-min walk test and a mild increase in LVEF. Despite localized injections, the left ventricular function seemed to improve globally.11
In our trial of 27 patients with idiopathic DC treated with intracoronary infusion of BMMNC, we observed no adverse effects and a clinical improvement in the NYHA functional class and NT-proBNP pro-brain natriuretic peptide levels. Although it could be argued that this improvement could be partly due to pharmacological treatment, it is important to note that all our patients were stable before entering the trial and medical treatment remained unchanged in most patients throughout the trial period. In addition, we observed a 6-month decrease in angiographic left ventricular volumes and a mean net gain of 9% in the LVEF. However, the gain in the LVEF after cell-therapy varied among patients. Two groups of patients were observed: responders and nonresponders. The responders were significantly younger. It is known that age may influence bone marrow functionality. The fat content in the bone marrow increases with age; aging also decreases the side population stem cells and granulocyte-macrophage colony forming units in bone marrow, as well as circulating CD34+, KDR+, and CD133 cells; and SDF-1 and IGF-1 levels in blood.21
Interestingly, the 24h migratory capacity of the cell suspension to VEGF and SDF-1 were significantly lower in the responders. Marrow cells that can migrate along a VEGF gradient in 24 h are almost all mature progenitors, while truly immature stem cells need several days to migrate. Thus, the gain in LVEF also seems to be enhanced by a lower migratory capacity of the cell suspension, with a higher percentage of immature infused cells. Fisher-Rasokat et al.9 also observed a significant inverse correlation between the extent of recovery in left ventricular hypokinesia and the functionality of the infused cells, as measured by their colony-forming capacity. These findings might imply a greater effect of the cell suspension when there are a higher proportion of undifferentiated cells with no migratory capacity or hematopoietic colony formations. Similar observations were seen in patients with chronic myocardial infarction treated with intracoronary infusion of BMMNC.5
Moreover, there was an inverse correlation between the gain in LVEF and the baseline HDL serum levels, suggesting a higher gain with lower HDL levels. In addition, baseline HDL levels also correlated inversely with the number of immature progenitors: CD34+ cells and CD117+ cells infused. The role of circulating HDL levels seems to be constantly growing. In vitro, cell-sgrowth and colony formation are inhibited in HDL-supplemented serum.22 In vivo, HDL is well known to promote the efflux of cholesterol from foam-cells, and it has anti-inflammatory and antiatherogenic properties.23,24 HDL contributes to antioxidant, vasodilating, and antithrombotic characteristics and modulates platelet activation.25 HDL even plays a role in the immune system.26 In addition, HDL plasma levels are related to the number of circulating endothelial progenitor cells.27 Tso et al.28 suggested a role for HDL in promoting progenitor mediated endothelial repair. In fact, low HDL is an independent predictor of endothelial dysfunction.29 At the bone marrow level, HDL is known to suppress hematopoietic stem cell proliferation.30 Conversely, lower HDL levels may play a role in promoting proliferation in patients with neoplastic processes.22,31 The unexpected relationship between the gain in LVEF and baseline HDL levels might suggest that low plasma HDL levels would represent a favourable environment for cell proliferation in the myocardium after the stimulus of BMMNC infusion. However, this needs to be confirmed in further studies with longer series of patients. Our findings also suggest a relationship between the bone marrow functionality status and the circulating HDL levels.
Limitations of the StudyThe number of patients in a single centre trial is limited and this was not a randomized trial. The main purpose of this trial was to ascertain safety and efficacy, both of which had favourable results. The number of patients considered to be nonresponders was small, but their responses to cell-therapy were clearly different and might be a point of interest for further studies.
CONCLUSIONSThis trial introduces the concept of responsiveness to cell-therapy in patients with DC. We observed a clinical and functional improvement in patients with DC after intracoronary cell-therapy. However, the responsiveness seems higher in younger patients and those with lower HDL plasma levels. In addition, the observed functional improvement seems to be enhanced by a lower migratory capacity of the infused cells.
FUNDINGThis clinical trial was supported by a grant (TCMR 007/06) from Fundación Progreso y Salud, Junta de Andalucía, Spain.
CONFLICTS OF INTERESTNone declared.