Hypertension is a risk factor for atrial fibrillation. Activation of the renin-angiotensin-system seems to be involved in atrial enlargement, with release of atrial and brain natriuretic peptides. The aim of this study was to evaluate the relationship between ambulatory blood pressure and levels of natriuretic peptides, with left atrial size in normotensives with idiopathic atrial fibrillation.
MethodsThis was a cross-sectional study in patients with idiopathic atrial fibrillation. The following measurements were recorded during the course of the study: office and 24-h ambulatory blood pressure, atrial and brain natriuretic peptides, plasma renin, aldosterone, and angiotensin–converting enzyme.
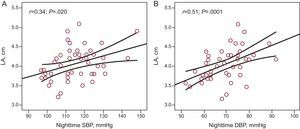
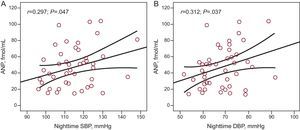
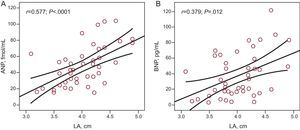
ResultsForty-eight patients (mean age 55 [10] years; 70.6% male) were included in the study. Mean office sitting blood pressure values were 132.49 (14.9)/80.96 (9.2) mmHg. Mean 24-h ambulatory systolic and diastolic blood pressure values were 121.10 (8.3)/72.11 (6.8) mmHg (daytime, 126.8 [9.7]/77.58 [7.9] mmHg; nighttime, 114.56 [11.6]/68.6 [8.8] mmHg). A clear trend towards increased left atrial size with higher ambulatory blood pressure values was noted, which was statistically significant for nighttime values (r=0.34; P=.020 for systolic and r=0.51; p=.0001 for diastolic). A significant correlation between atrial natriuretic peptide and nighttime systolic (r=0.297; P=.047) and diastolic (r=0.312; P=.037) blood pressure was observed. Significant correlations were also observed between left atrial size and atrial natriuretic peptide levels (r=0.577; p<.0001) and brain natriuretic peptide levels (r=0.379; P=.012).
ConclusionsNighttime blood pressure is associated with left atrial size and the release of natriuretic peptides in normotensive patients with idiopathic atrial fibrillation.
Keywords
.
INTRODUCTIONAtrial fibrillation (AF) is the most common cardiac arrhythmia in the general population, and its prevalence is increasing in direct proportion to the aging of the population.1 Recent data from the Val-FAAP study2 (patients with AF in a primary care setting), an epidemiological study conducted in primary care centres in Spain, reported a 6.1% prevalence of AF among subjects. However, other studies conducted in the general population of Spain have shown a reduced prevalence of AF (2.3%) in subjects older than 18 years of age.3 In 70%-80% of the cases, the onset of AF is associated with demonstrable structural heart disease (valvular or ischemic), hypertension, diabetes mellitus, or thyroid disease.4 The 20%-25% of cases that occur in people without apparent organic disease are defined as idiopathic or isolated atrial fibrillation (IAF).5 Previous studies have only found an increased anteroposterior diameter of the left atrium in patients with IAF as compared to the general population6; this condition could act as an anatomical substrate favourable for the development of IAF.7
Epidemiological studies have shown that hypertension is the most important risk factor involved in the development of AF.8 It promotes morphological and functional alterations including left ventricular hypertrophy, concentric left ventricle remodelling, diastolic dysfunction, and increased left atrial diameter.9 In recent years, biomarkers have been identified that correlate with structural heart lesions, including atrial natriuretic peptides (ANP) and brain natriuretic peptides (BNP), although the mechanism by which these natriuretic peptides are elevated in hypertension remains unclear.10 Some authors suggest that elevated natriuretic peptides, specifically ANP, are more closely associated with blood pressure (BP) per se, than with ventricular remodelling secondary to hypertension.11
Recent studies suggest that activation of the renin-angiotensin-aldosterone system (RAAS) plays an important role in the development of AF through 2 main mechanisms: a) by promoting myocardial fibrosis, primarily at the atrial level, thereby acting as a potentially arrhythmogenic anatomical substrate,12 and b) by inducing structural changes such as left ventricular hypertrophy and atrial enlargement, which are also responsible for the neurohormonal activation involved in the development of IAF.13–15
The objective of this study was to test the hypothesis that ambulatory BP values are associated with structural cardiac abnormalities such as atrial enlargement, and with neurohormonal markers in normotensive subjects with IAF.
METHODSDesign and Study PopulationWe carried out a cross-sectional study of a cohort of patients diagnosed with IAF in the GIRAFA (Grup Integrat de Recerca en Fibril·lació Auricular) study (integrated group for research in AF), at the Hospital Clinic of Barcelona (Spain), between January 2001 and June 2005. IAF was defined as a symptomatic episode of paroxysmal AF without structural heart disease demonstrable by 2-dimensional echocardiography or other secondary causes of AF such as an established diagnosis of hypertension, diabetes mellitus type 2, chronic obstructive pulmonary disease, chronic liver disease, or inflammatory or infectious disease. Patients with AF secondary to drug addiction, hypoxemia, alcohol abuse, or thyroid disease were also excluded. Patients were included consecutively after telephone contact. All patients were included after giving their written informed consent. The protocol was approved by the Hospital Clinic of Barcelona ethics committee.
Clinic and Ambulatory Blood Pressure MeasurementPatients attended the hospital on a weekday between 8:00 a.m. and 10:00 a.m. BP was measured according to the European Society of Hypertension/European Society of Cardiology 2007 guidelines.16 After 5min of rest, 3 BP measurements were taken in a sitting position, each measurement separated by 2min. Patients with mean BP>140/90mmHg were excluded from the study. Ambulatory BP measurement/monitoring (ABPM) was done using a Spacelabs 90207/90217 device (Spacelabs® Inc.; Richmond, Washington, United States), with readings scheduled every 20min during the 24-h period, after the usual recommendations to the patients. The periods of activity and rest were determined on an individual basis according to hours of sleep and waking. The duration of ABPM in hours, the percentage of valid readings, and mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) during periods of activity and rest, and for the whole 24-h period were measured. Records with a duration n<24h, those lacking ≥1 reading per hour, and those with <80% satisfactory readings, were excluded. According to the European Society of Hypertension/European Society of Cardiology 2007 guidelines definition for 24-h ABPM; BP levels <130/80mmHg were considered as normal.
Analytical DeterminationsBlood samples were extracted between 9.00 a.m. and 11.00 a.m., with the patient in a supine position, and after 30min to 45min of rest. BNP level in plasma was determined by chemiluminometric immunoassay using an ADVIA Centaur immunochemistry analyser (Siemens Healthcare Diagnostics; Tarrytown, New York, United States). Plasma renin activity was determined by radioimmunoassay (GammaCoat Plasma Renin Activity 125I RIA Kit, DiaSorin; Stillwater, Mississippi, United States). Angiotensin-converting enzyme was measured in serum by a kinetic test (Bühlmann Laboratories AG; Schonembuch, Switzerland). Plasma aldosterone was determined using a standard radioimmunoassay (Coat-A-Count Aldosterone, Diagnostic and Products Corporation; Los Angeles, California, United States). ANP was determined by plasma extraction using Cl8 Sep-Pack cartridges (Waters Associates; Milford, Massachusetts, United States) and subsequently, radioimmunoassay was performed using a commercial kit (Euro-Diagnostica B.V; Arnhem, The Netherlands).
EchocardiogramTransthoracic echocardiography was performed using validated devices (Philips IE-33) and images were digitized for further analysis. All measurements were performed in M-mode and 2-dimensional with patients in sinus rhythm, as recommended by the American Society of Echocardiography.17 The size of the left atrium was determined using anteroposterior (parasternal), transverse (mediolateral), (mediolateral), and longitudinal (inferosuperior) views. We also determined the thickness of the posterior wall, the thickness of the interventricular septum, and left ventricular end-diastolic diameter in millimetres, and left ventricular mass in grams. We calculated the left ventricular mass index adjusted for body surface area in g/m2, and the relative (ventricular) wall thickness using the formula: 2 times the thickness of the posterior wall/left ventricular end-diastolic diameter. Concentric left ventricular hypertrophy was defined as left ventricular mass index >125g/m2 in men or >110g/m2 in women with relative (ventricular) wall thickness>0.45. Concentric remodelling was defined as relative (ventricular) wall thickness>0.45 with normal left ventricular mass index.14,15
Statistical AnalysisClinical characteristics and variables with a normal distribution were expressed as mean (standard deviation). Continuous variables were analyzed using the Student t test and categorical variables using the chi-square test. The Kolmogorov-Smirnov and the Saphiro-Wilk tests were carried out to evaluate whether continuous variables had a normal distribution. Only BNP did not have a normal distribution (P=.001). Hence, simple correlations were made using the Spearman's correlation coefficient.
To analyze the relationship between continuous variables, linear multivariate regression analysis was carried out using linear sequential steps with a confidence interval of 95%. Linearity assumption was tested graphically. A value of P<.05 was considered statistically significant. Finally, all statistical analyses were performed using the SPSS v11.0 statistical package (SPSS Inc.; Chicago, Illinois, United States).
RESULTSOf the original sample of 107 patients with paroxysmal AF included in the GIRAFA study, only 60 patients agreed to be included in the present study after a telephone call; of these, 3 patients were excluded from the study due to concomitant beta-blocker therapy, 5 patients due to BP values >130/80mmHg in 24-h ABPM, and 4 patients due to invalid ABPM readings. Therefore, 48 strictly normotensive patients never treated with antihypertensive drugs before, were finally analyzed in the study.
Mean age of the patients was 55.41 (10) years and 70.6% were male. No patient had received previous antihypertensive treatment; 23.6% patients had received antiarrhythmic therapy (16.7% patients received flecainide and 5.6% patients received amiodarone) and 51.7% patients had received antiplatelet therapy (24% patients received aspirin, 3.5% patients received dipyridamole, and 24.1% patients received acenocoumarol). Mean clinic SBP/DBP was 132.45 (14.9)/80.96 (9.20) mmHg. No patient presented sustained clinic BP>140/90mmHg. Mean ambulatory SBP/DBP in the 24-h period was 121.10 (8.3)/72.11 (6.8) mmHg; daytime was 126.8 (9.7)/77.58 (7.9) mmHg and nighttime was 114.56 (11.6)/68.6 (8.8) mmHg.
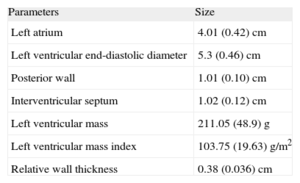
Table 1 shows the main echocardiographic data. Only the left atrial size, which was in the upper normal range, with a mean diameter of 4±0.42cm, was remarkable.
Echocardiographic Parameters.
| Parameters | Size |
| Left atrium | 4.01 (0.42) cm |
| Left ventricular end-diastolic diameter | 5.3 (0.46) cm |
| Posterior wall | 1.01 (0.10) cm |
| Interventricular septum | 1.02 (0.12) cm |
| Left ventricular mass | 211.05 (48.9) g |
| Left ventricular mass index | 103.75 (19.63) g/m2 |
| Relative wall thickness | 0.38 (0.036) cm |
Data are expressed as mean (standard deviation).
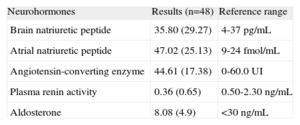
Table 2 shows mean (standard deviation) levels of ANP, BNP, angiotensin-converting enzyme, plasma renin, plasma aldosterone, and the applicable reference values. BNP levels were in the upper limit of normality, and mean ANP levels were 2 times higher than the reference range.
Neurohormonal Tests.
| Neurohormones | Results (n=48) | Reference range |
| Brain natriuretic peptide | 35.80 (29.27) | 4-37 pg/mL |
| Atrial natriuretic peptide | 47.02 (25.13) | 9-24 fmol/mL |
| Angiotensin-converting enzyme | 44.61 (17.38) | 0-60.0 UI |
| Plasma renin activity | 0.36 (0.65) | 0.50-2.30 ng/mL |
| Aldosterone | 8.08 (4.9) | <30 ng/mL |
Data are expressed as mean (standard deviation).
There was a clear trend towards greater left atrial size in patients with higher 24-h ABPM levels (r=0.24; P=.11), and there was a significant association between left atrial size and nighttime ambulatory SBP (r=0.34; P=.020) (Fig. 1A), and DBP (r=0.51; P=.0001) (Fig. 1B). There was a significant correlation between plasma ANP levels and nighttime ambulatory SBP (r=0.297; P=.047) (Fig. 2A) and DBP (r=0.312; P=.037) (Fig. 2B). There was no correlation between levels of ANP and daytime BP or between ambulatory BP values and levels of BNP or aldosterone.
There was a statistically significant correlation between left atrial size and ANP levels (r=0.577; P<.0001) (Fig. 3A) and BNP levels (r=0.379; P=.012) (Fig. 3B). There was no correlation between levels of neurohormonal markers and other echocardiographic parameters analyzed, except for interventricular septum thickness and plasma aldosterone levels.
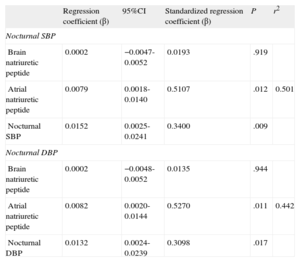
Finally, the multiple regression analysis showed that for each centimetre of increase in size of the left atrium, nocturnal SBP increased by 15mmHg (P=.009) and nocturnal DBP increased by 13mmHg (P=.017), independently of neurohormonal markers (Table 3).
Multiple Regression Analysis With Left Atrium Size as Dependent Variable for Nocturnal Systolic Blood Pressure and Nocturnal Diastolic Blood Pressure.
| Regression coefficient (β) | 95%CI | Standardized regression coefficient (β) | P | r2 | |
| Nocturnal SBP | |||||
| Brain natriuretic peptide | 0.0002 | −0.0047-0.0052 | 0.0193 | .919 | |
| Atrial natriuretic peptide | 0.0079 | 0.0018-0.0140 | 0.5107 | .012 | 0.501 |
| Nocturnal SBP | 0.0152 | 0.0025-0.0241 | 0.3400 | .009 | |
| Nocturnal DBP | |||||
| Brain natriuretic peptide | 0.0002 | −0.0048-0.0052 | 0.0135 | .944 | |
| Atrial natriuretic peptide | 0.0082 | 0.0020-0.0144 | 0.5270 | .011 | 0.442 |
| Nocturnal DBP | 0.0132 | 0.0024-0.0239 | 0.3098 | .017 | |
95%CI, 95% confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
The primary finding of this study was that, in normotensive subjects never treated with antihypertensive drugs suffering with IAF, nighttime ambulatory BP was directly associated with the size of the left atrial wall and with plasma levels of ANP and BNP.
Epidemiological studies have shown that hypertension is associated with an increased risk of AF. High BP induces structural and functional alterations, mainly in the form of left atrial enlargement.7 Therefore, left atrial size is a recognized risk factor for AF and stroke.18 Studies have shown a linear relationship between clinic BP and left atrial size,19 although, published data on ambulatory BP levels are limited, particularly in normotensive subjects. Evidence to date, suggests that patients with essential hypertension have a higher atrial volume than normotensive subjects and that these differences appear to be more marked in patients without a reduction in nighttime BP, the so-called non-dipper circadian pattern.20 Our results are in line with those of Cuspidi et al.21 who found a modest, but significant, correlation between left atrial size and nighttime ambulatory BP values in hypertensive subjects.
Recent years have provided much evidence indicating that a reduction in the nighttime BP is associated with an increase in subclinical organ damage22 and cardiovascular complications. Therefore, nighttime BP is considered the best predictor of cardiovascular events, and is independent of daytime ambulatory BP values and the dipper/non-dipper circadian pattern.23,24 Nighttime BP levels, and not office BP levels, play the most important role in left atrial remodelling and enlargement, possibly due to RAAS activation. Some authors suggest that RAAS activation induces a series of electrical and structural changes in the left atrium that act as an anatomical substrate favouring the development of AF.13,14 Studies have shown that angiotensin II is involved in the regulation of cardiomyocyte proliferation and collagen deposition25 in the interstitial matrix. Therefore, RAAS activation may act as a promoter of growth and tissue fibrosis in the myocardium. Studies suggest that atrial fibrosis is potentially arrhythmogenic, facilitating re-entry stimuli at this level,13 which would predispose an individual to the development of IAF. The structural alterations induced by RAAS activation, together with the anatomic alterations secondary to the potent arterial vasoconstriction induced by the action of angiotensin II (such as ventricular hypertrophy and left atrial enlargement), may result in a dilated, potentially arrhythmogenic left atrium in an apparently normotensive patient. However, RAAS activation in normotensive subjects, such as our patients, is very low and; significant increases in angiotensin II, plasma renin activity, and aldosterone levels have not been observed. The design of our study cannot answer the relevant question of what is first, the rise in BP (even in the normal range) or the activation of the RASS, although the latter would be a more logical explanation.
In recent years, there has been progress in the search for biomarkers with a high predictive value in cardiovascular disease, such as cardiac natriuretic peptides (BNP and ANP). Previous studies have shown that vasoactive peptides are elevated in patients with hypertension, although the results are contradictory. On the 1 hand, it is postulated that high ABPM levels are more closely associated with levels of BNP and ANP than clinic BP.26 Furthermore, recent studies have shown that elevations in vasoactive peptides are more closely related to BP levels than to the growth of the myocardial mass itself and; a significant correlation between SBP levels and ANP have been found in these studies (r=0.34; P=.05).11 We found a significant direct correlation between nighttime BP and plasma levels of ANP, both for SBP (r=0.297; P=.047) and for DBP (r=0.31; P=.037). These results provide support for the study by Jensen et al.,27 who found that the non-dipper circadian pattern was associated with an increase in the release of vasoactive hormones.
LimitationsThe study has some limitations, of which the small sample size is undoubtedly the most important. Also, a control group could have added to the robustness of our findings. Larger, case-controlled studies will be needed in the future to confirm our results.
CONCLUSIONSThe results of this study confirm the importance of nocturnal BP values, which even when in the normal range, are associated directly with the size of the left atrial wall and the release of ANP and BNP in patients with IAF.
CONFLICTS OF INTERESTNone declared.