The Fontan operation is usually the final palliative procedure in patients with univentricular heart. The objectives of this study were, firstly, to describe the clinical and haemodynamic characteristics of a group of patients with univentricular physiology who had previously been palliated with a bidirectional Glenn procedure and, secondly, to identify risk factors that can influence postoperative outcomes after the Fontan operation.
MethodsRetrospective study with 32 patients who underwent a Fontan operation between March 2000 and December 2009. Clinical characteristics, catheterization data, type and duration of surgery were revised and analyzed as predictors of postoperative outcome.
ResultsHospital mortality was 3%. After a median follow-up of 44 months (interquartile range, 32-79), survival was 90%. Preoperative mean pulmonary arterial pressure (measured during catheterization) was correlated with late mortality. Of the remaining variables analyzed, the Nakata and McGoon indices, and duration of cardiopulmonary bypass showed the highest correlations with postoperative outcomes. Interventional catheterization before the Fontan operation was performed in 42% of patients.
ConclusionsHospital mortality after the Fontan operation was very low. The performance of a haemodynamic study before the Fontan operation made it possible to select high-risk patients for surgery as well as permitting the performance of interventional procedures that could improve postoperative outcome in these patients.
Keywords
.
IntroductionPatients with a functionally univentricular heart undergo a series of interventions intended to passively direct systemic venous return toward pulmonary circulation, thereby leaving the single ventricle to boost systemic circulation. This is known as the Fontan situation or physiology.
For this delicate system to function correctly, good systolic and diastolic functioning is required, together with the presence of sinus rhythm, low pulmonary pressure and vascular resistance, and appropriate respiratory tree anatomy.1 Ever since the Fontan technique was first introduced, cardiac catheterization has been considered a necessary part of pre-intervention evaluation.
There have been numerous modifications to the technique since it was first described by Fontan in 1971.2 One such modification is the performance of an anastomosis between the superior vena cava and the pulmonary artery (bidirectional Glenn operation). This intermediate step is intended to stabilize pulmonary flow and avoid an overly heavy flow through the ventricle.3 Other modifications include the abandonment of the atriopulmonary connection in favor of a Fontan-type lateral tunnel,4 the use of extracardiac conduits,5 and the application of baffle fenestration.6
Methods PatientsBetween March 2000 and December 2009, 32 patients with univentricular physiology who had previously received a bidirectional Glenn operation underwent Fontan surgery at our center. Background data collected included demographic information, type of heart disease, systemic ventricular anatomy, presence of heterotaxy, neonatal surgery type, age at which the bidirectional Glenn operation was performed, systemic atrioventricular valve regurgitation, and ventricular dysfunction by echocardiography (the last two conditions were dichotomized into “non-mild” and “moderate to severe”). Patient characteristics are shown in Table 1.
Table 1. Patient Characteristics.
| Demographic data | |
| Age at which Glenn performed, months | 8.5 [5-13.3] |
| Age at which Fontan performed, years | 5.3 [4.2-6.4] |
| Time between Glenn and Fontan, months | 52.5 [41-65.5] |
| Sex (males/females) | 15/17 |
| Weight at Fontan, kg | 17.250 [16-19] |
| Body surface, m2 | 0.68 [0.64-0.74] |
| Type of heart disease | |
| Tricuspid atresia | 10 (31) |
| HLHS | 7 (22) |
| Single ventricle | 7 (22) |
| Doble outlet right ventricle | 4 (13) |
| Unbalanced AV canal | 2 (6) |
| D-TGA, IVC and PS | 1 (3) |
| Ebstein anomaly | 1 (3) |
| Heterotaxy | 4 (13) |
| Type of ventricle | |
| Right | 12 (38) |
| Left | 17 (53) |
| Two ventricles | 3 (9) |
| Neonatal surgery | |
| Blalock-Taussig shunt | 13 (41) |
| Norwood | 8 (25) |
| Other surgery | 3 (9) |
| No neonatal surgery | 8 (25) |
| Moderate to severe AV valve regurgitation | 5 (16) |
| Moderate-severe ventricular dysfunction | 2 (6) |
AV, atrioventricular; D-TGA, D-transposition of great arteries; HLHS, hypoplastic left heart syndrome; IVC, interventricular communication; PS, pulmonary stenosis.
Data are expressed as no. (%) or medians [interquartile range].
All patients were catheterized in the 6 months prior to the Fontan procedure. None of the catheterized patients were excluded from the Fontan operation based on hemodynamic data. Catheterization was performed under general anesthesia using endotracheal intubation. Patients were disconnected from the respirator when pressures were measured. Pressures were measured and oximetry performed in the superior vena cava, the pulmonary arteries, the left atrium, the systemic ventricle, and the aorta. We used these data to calculate pulmonary flow and systemic and pulmonary vascular resistance based on published formulas.7 Postoperative pressure in the Fontan was estimated using the following formula8:
All angiograms were reviewed and pulmonary artery diameters were measured and the results used to calculate the Nakata9 and McGoon indices as well as the lower lobe index. The findings of the Spicer et al. study10 were used to classify the degree of collateral circulation. Pulmonary branch distortion was deemed to be present when there was stenosis of >50% in branch caliber with respect to an adjacent segment or when there was marked hypoplasia of the branches.11 Significant venovenous collaterals were deemed to be present when diameter was >2mm.12
Surgical ProceduresWe collected data on the surgical technique used (extracardiac or lateral tunnel Fontan, surgery with cardiopulmonary bypass [CPB], aortic clamping and / or circulatory arrest), surgical times, and associated procedures. Fontan fenestration was performed by direct anastomosis when the lateral tunnel approach was used and through a GORE-TEX® conduit in extracardiac cases.
Postoperative Variables and Follow-UpPostoperative Fontan pressure was measured using a catheter inserted via the jugular vein into the superior vena cava. Left atrial pressure was measured with a transthoracic catheter. TPG was calculated. We collected data on duration of effusions, mechanical ventilation times, length of stay in the pediatric intensive care unit (PICU), and length of hospital stay, and we studied their relationship with clinical, hemodynamic, and surgical variables. We also recorded immediate postoperative complications and hospital and post-discharge mortality. After discharge, all patients were reviewed clinically in the hospital outpatient clinic at least once a year using echocardiography and electrocardiography. No patients were lost to follow up.
Statistical AnalysisData were collected retrospectively from medical records. Continuous variables were expressed as means (standard deviations) or medians [interquartile range]. Categorical variables are shown as frequencies and percentages. Student's t test was used for between-group comparisons with normally distributed continuous variables. The Mann-Whitney test was used for variables showing a non-normal distribution. Linear regression was used to analyze correlations between continuous variables. Comparisons between categorical variables were performed using the χ2 test or Fisher's exact test. Data were analyzed using SPSS 15.0 (SPSS Inc., Chicago, Illinois, United States).
Results Clinical VariablesNone of the patient characteristics studied influenced postoperative evolution of the Fontan operation.
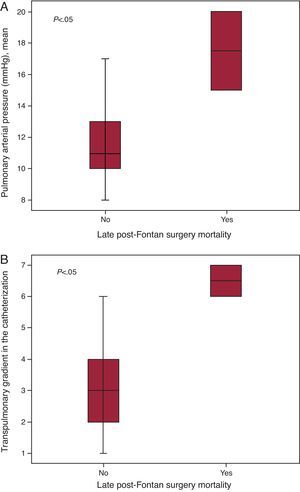
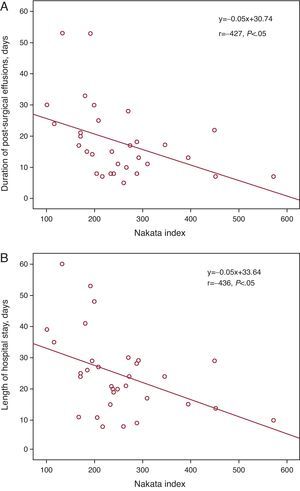
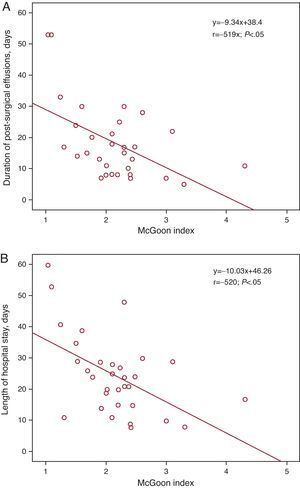
Cardiac Catheterization Before the Fontan OperationCatheterization was purely diagnostic in 19 patients (59%), but was interventional (one or more interventions) in 13 (41%) patients (Table 2). None of the patients died or suffered serious complications following catheterization. Data derived from cardiac catheterization are shown in Table 3. Mean pulmonary arterial pressure (mPAP) and the TPG measured during catheterization were significantly higher in patients who died later compared to survivors (median mPAP of the deceased and the survivors, 17.5 [15-20] vs 11 mmHg [10 to 13.2], P<.05); TPG, 6.5 [6-7] vs 3 mmHg [1.75-4], respectively, P<.05) (Figure 1). Patients with mPAP >15mmHg stayed longer in PICU (10.5 [4.2]days) than those with mPAP ≤15mmHg (5.9 [4.3]days) (P<.05). Both the Nakata and the McGoon indices were inversely correlated with length of hospital stay and duration of effusions (Figure 2, Figure 3).
Table 2. Interventional Procedures Prior to Fontan.
| no. (%) | |
| Angioplasty of the pulmonary branches | 7 (22) |
| Embolization of aortopulmonary collaterals | 4 (13) |
| Embolization of venovenous collaterals | 3 (9) |
| Antegrade pulmonary flow embolization | 1 (3) |
| Aortic angioplasty | 1 (3) |
Table 3. Pre-Fontan Catheterization Data.
| Hemodynamic study | |
| mPAP, mmHg | 11,5 [10-14] |
| VEDP, mmHg | 10 [9-12,7] |
| PsA, mmHg | 9 [7-10,7] |
| iPAR, WU·m2 | 0,9 [0,5-1,2] |
| O2 aortic saturation, % | 86 [80-90] |
| Qp/Qs | 0,53 [0,45-0,66] |
| Nakata index, mm2/m2 | 236 [189-288] |
| McGoon index | 2,1 [1,6-2,4] |
| Lower lobe index, mm2/m2 | 124 [102-166] |
| Distortion of pulmonary branches | 7 (22) |
| Aortopulmonary collaterals | |
| Grade 1 | 0 |
| Grade 2 | 3 (9) |
| Grade 3 | 17 (53) |
| Grade 4 | 12 (38) |
| Venovenous collaterals | 19 (59) |
iPAR, indexed pulmonary arteriolar resistance; mPAP, mean pulmonary arterial pressure; PsA, systemic atrial pressure; Qp/Qs, pulmonary/systemic flow ratio; VEDP, ventricular end-diastolic pressure; WU: Wood units.
Data are expressed as no. (%) or medians [interquartile range].
Figure 1. Box plot of mean pulmonary arterial pressure (A) and transpulmonary gradient (B) measured in pre-Fontan catheterization in patients who died late after surgery and in survivors.
Figure 2. Linear regression lines between the Nakata index and duration of effusions (A) and length of hospital stay (B).
Figure 3. Linear regression lines between the McGoon index and the duration of effusions (A) and length of hospital stay (B).
SurgerySurgical procedures and times are shown in Table 4. There was a very strong correlation between duration of CPB and intubation time (r=.734, P<.001), and between duration of CPB and length of stay in PICU (r=.566, P<.05). CPB time was also significantly higher in those who had effusions lasting more than 14 days (162[62]min) compared to those with effusions lasting under 14 days (123[31]min) (P<.05).
Table 4. Procedures and Surgery Times.
| Type of Fontan | |
| Extracardiac conduit | 25 (78) |
| Lateral tunnel | 7 (22) |
| Fenestration | 12 (38) |
| CPB | 29 (91) |
| Aortic clamp | 11 (35) |
| Circulatory arrest | 4 (13) |
| Surgery times | |
| CPB time (min) | 143±51 |
| Aortic clamp time (min) | 78±37 |
| Associated surgical procedures | |
| Pulmonary artery ligation | 3 (9) |
| Pulmonary branch plasty | 3 (9) |
| Enlargement of bulboventricular foramen | 1 (3) |
| Tricuspid plasty | 1 (3) |
| Repair of aortic arch | 1 (3) |
CPB, cardiopulmonary bypass.
Data are expressed as no. (%) or mean±standard deviation.
Length of stay in PICU, mechanical ventilation, and duration of CPB were significantly higher in patients in which fenestration was used (9.1[6]days, 75.6[105.7]h, 184[48]min) compared to non-fenestrated patients (5[2.5]days, 16.5[13.6]h, 119[37]min, respectively) (P<.05). The pressure in the Fontan, as estimated using the formula described in the “Methods” section, was also significantly higher in patients in which the Fontan was fenestrated (18.1[5.2]vs 11.5[1.6]mmHg) (P<.005).
MortalityHospital survival was 97%. After a median follow up of 44 months [32-79], survival was 90%. One patient died in the immediate postoperative period. The patient had a hypoplastic left heart syndrome and significant desaturation due to the presence of pulmonary arteriovenous collaterals and an aortic coarctation. At the age of 3.5 years, the patient underwent an extracardiac Fontan procedure with fenestration and aortic arch repair. As it was not possible to take the patient off the pump, an extracorporeal membrane oxygenator was used to assist him from the operating theatre. The patient was then weaned off the oxygenator. However, he was subsequently found to have a severe hypoxic-ischemic encephalopathy and the appropriate therapeutic strategies were applied. Another patient died 3 months after surgery. This patient had heterotaxy syndrome with a single atrioventricular valve, single ventricle, and pulmonary stenosis. At 18 months, the patient underwent a lateral tunnel Fontan operation without fenestration. After an uncomplicated postoperative period, the patient began to show signs of venous congestion at 3 months after surgery. Catheterization indicated some raised ventricular end-diastolic and pulmonary pressures. Medical treatment was started, but the patient died from failure of the Fontan. A third patient with D-transposition of the great arteries, unrelated ventricular septal defect, and pulmonary stenosis complex died 3 years after the Fontan. This patient underwent extracardiac Fontan with fenestration at the age of 6 years. After an uncomplicated early postoperative period, the patient began to have difficult-to-control focal seizures secondary to a subdural empyema. The patient then began to show signs of lower lobe atelectasis and died after a diaphragmatic plication and resection of a lung lobe.
Postoperative PeriodPostoperative variables and complications are shown in Table 5. Fontan pressure was correlated with postoperative length of stay in PICU (r=.67, P<.001), duration of effusions (r=.404, P<.05), and length of hospital stay (r=.611, P<.001).
Table 5. Postoperative Outcomes.
| Postoperative variables | |
| Mean Fontan pressure, mmHg | 11.5 [10-14] |
| Intubation time, h | 18 [7-36] |
| Length of stay in PICU, days | 5 [4-10] |
| Duration of pleural effusions, days | 14 [8-22] |
| Length of hospital stay, days | 24 [15-29] |
| Mortality | |
| Hospital | 1 (3) |
| Late | 2 (6) |
| Early postoperative complications | |
| Infection * | 19 (59) |
| Arrhythmias | 8 (25) |
| Sinus node dysfunction | 3 (11) |
| Nodal tachycardia | 3 (11) |
| Flutter | 2 (6) |
| Neurological complications | 3 (9) |
| HIE | 1 (3) |
| Subdural empyema | 1 (3) |
| Embolic stroke | 1 (3) |
| Reinterventions | 7 (22) |
| Fontan fenestration | 3 (9) |
| Plication of the diaphragm | 2 (6) |
| Pulmonary branch plasty | 1 (3) |
| Bleeding | 1 (3) |
| Aortic arch repair | 1 (3) |
| Early postoperative catheterization | 6 (19) |
| Diagnostic | 2 (6) |
| Pulmonary branch stent | 2 (6) |
| Embolization of aortopulmonary collaterals | 3 (9) |
| Antegrade pulmonary flow embolization | 1 (3) |
HIE, hypoxic-ischemic encephalopathy; PICU, pediatric intensive care unit.
Data are expressed as no. (%) or medians [interquartile range].
* Bacteraemia, mediastinitis or superficial wound infection.
Patients with univentricular physiology who are eventually palliated with Fontan surgery form a heterogeneous group. They have different underlying congenital heart diseases and often undergo other surgical interventions before the Fontan operation; there are also notable differences in the techniques used, and the age at which they undergo a Fontan procedure varies. This heterogeneity leads to differences in preoperative risk factors between different surgical groups, as well as changes in those risk factors over time.
The age at which the Fontan procedure is performed is one of the factors which has, over the years, been linked to patient evolution. Early studies indicated that performing the Fontan operation at a younger age was a major risk factor for mortality, though the finding was probably related to the fact that surgical techniques were in their infancy.11, 13 Over time, the trend has been toward performing the intervention earlier, with no apparent increase in mortality.14 Theoretical advantages of earlier intervention include a reduction in the body's exposure to cyanosis, and its negative effect on cardiac function,15 and partial avoidance of aortopulmonary collateral formation, which some studies have shown to be associated with increased pulmonary pressure and longer duration of Fontan effusions.10, 16, 17 Earlier intervention could also lower Fontan pressure, an important consequence given that most series have shown that higher pressure is associated with a increased risk of death and early postoperative failure.11, 13, 18 Although patients in our series were somewhat older than those in most modern series, the low hospital mortality rate (3%) was similar to that reported in a recent study14 and lower than reports from older series.18, 19 However, the fact that Fontan was performed relatively late in our series explains why up to 50% of patients had prolonged effusions (>14 days). Our study also confirms the previously described relationship between pressure in the Fontan and duration of effusions.20
In most of the initial series, mPAP and pulmonary vascular resistance were found to be key risk factors for mortality and Fontan failure.11, 13, 18, 19 However, in more recent series, pre-Fontan hemodynamic data did not appear to affect short-term postoperative outcomes.12, 14, 21, 22 This is probably related to advances in patient preparation in the pre-Fontan stage. One such advance is the performance of the Glenn operation at an earlier age, an approach which avoids volume-loading and secondary hypertrophy in the single ventricle and helps to preserve its diastolic function, any alteration in which can critically affect circulation in the Fontan.14, 15 In our series, mean pulmonary pressure was associated with late mortality. Some studies have indicated that progressive pulmonary vascular disease occurs after the Fontan operation due to lack of flow pulsatility, causing complications and long-term mortality.23
It is still a matter for debate whether the size of the pulmonary arteries, as measured in the pre-Fontan catheterization and normalized using the Nakata and McGoon indices and the indexed area of the lower lobe, is a prognostic factor for later clinical course in the Fontan operation. Some series have shown that these indices have a clear effect on postoperative outcomes,9, 24 while others have suggested that they have no such effect.25, 26 The greatest contribution to pulmonary resistance is from the precapillary arterioles, then the post-capillary venules, so it is not surprising that the size of the central pulmonary arteries is not a factor. Numerous studies have sought to establish the lowest limit of the pulmonary arteries compatible with the completion of a Fontan operation. A Nakata index of >250mm2/m2 was initially proposed,9 but the limits have been continually revised downwards since then, and a recent study27 based on a theoretical model of Fontan established that a Nakata index of 110mm2/m2 was the lowest value compatible with a pressure of <17mmHg during exertion. Because it is not affected by previous surgeries, some authors propose using the lower lobe index.28 We found an inverse correlation between the Nakata and McGoon indices and the duration of effusions and hospital stay, but not with the indexed area of the lower lobe.
With regard to the influence of specific surgical factors on postoperative Fontan outcomes, both CPB time and clamping have shown a clear association with early and late mortality in most series.11, 14, 19 Longer CPB and clamp times have a negative effect on cardiac function and pulmonary vasculature, both of which are critical to maintaining hemodynamic stability after any heart surgery, though they acquire much greater significance after the Fontan procedure. Some groups have opted for completing the extracardiac Fontan procedure without CPB29 and have already reported good results, with significant reductions in time on mechanical ventilation, time in PICU, and length of hospital stay compared to Fontan patients in which CPB was used.30 In our series, CPB time correlated with the duration of effusions and duration of mechanical ventilation, as well as with length of stay in PICU. We conducted off-pump Fontans in 3 patients (9%) and observed decreased length of stay in PICU, decreased length of hospital stay, and reduced duration of effusions, although the small number of cases meant that the differences were not statistically significant (data not shown).
Another of the controversies currently surrounding Fontan surgery is whether or not prior catheterization is required. Those who argue that catheterization is not necessary suggest that magnetic resonance imaging provides anatomical information of similar quality.31 This idea has been reinforced by the fact that most modern series show no significant relationship between hemodynamic data obtained during catheterization and postoperative course12, 14 and by the fact that the Fontan procedure is contraindicated in only a very few patients based on catheterization data. In our series, however, pulmonary pressure measured during catheterization was associated with late mortality and postoperative pressure of >15mmHg was associated with the performance of a fenestration in the Fontan. In our opinion, catheterization before Fontan provides valuable data that allows for the selection of at-risk patients in which measures such as Fontan fenestration or the pre- or post-operative administration of pulmonary vasodilators32, 33 can improve prognosis.
Another interesting feature of pre-Fontan catheterization is the possibility of performing different types of intervention. In a recent series from the Boston group (which advocates not performing pre-Fontan hemodynamic studies in selected cases), interventions were performed in up to 64% of cases, although in retrospect they were only considered important in 37% of patients.12 In our series, interventions during pre-Fontan catheterization were carried out in 42% of patients. If we add to these the 3 patients in which post-operative embolization of collaterals was performed to reduce the amount and duration of effusions, the proportion increased to 50%.
There is also considerable controversy among groups regarding the management of aortopulmonary collaterals. Some centers have reported that the presence of significant aortopulmonary collaterals was associated with increased pulmonary pressure and longer duration of effusions after Fontan.10, 16, 17 In contrast, other studies, such as that by McElhinney et al.34 and Bradley et al.35 found no significant effect of aortopulmonary collaterals, or embolization, on pulmonary pressure and duration of effusions. Some proponents of aggressive embolization of collaterals may argue that these studies did not completely occlude the collaterals and that the multiple connections between the internal mammary, lateral thoracic, thyrocervical trunk, and intercostal arteries mean that if only the proximal part of these vessels is occluded, a collateral revascularization would be produced from distal connections between these vessels. They therefore propose embolizing the entire vessel.36 Several studies have quantified the flow in the aortopulmonary collaterals. Using magnetic resonance imaging, one such study calculated that collaterals accounted for 46% of pulmonary flow and 36% of cardiac output after a bidirectional Glenn operation. The same study showed that collateral flow was higher the older the child at the time of the Fontan operation.37 In our series, almost 90% of patients had significant (grade 3 and 4) aortopulmonary collaterals consistent with the relatively late performance of the Fontan operation. This explains the prolonged drainage time in half of our patients. In our opinion, the possibility of performing interventional procedures is another reason for catheterization before the Fontan operation.
Study Limitations and StrengthsThe main limitations of this study were its retrospective nature and small sample size. Its principal strength lay in the fact that the series was relatively homogeneous and that all patients were catheterized, operated, and managed postoperatively using techniques and protocols which included only minor variations throughout the study period.
ConclusionsWe performed the Fontan operation with very low hospital mortality. Elevated pulmonary pressure in the pre-Fontan catheterization was an excellent predictor of late mortality. The correlation of the hemodynamic parameters (mPAP, TPG, estimated Fontan pressure, and the Nakata and McGoon indices) with postoperative outcomes indicates that the performance of cardiac catheterization before Fontan is a good method for selecting high risk patients and implementing measures to improve outcomes. In addition, catheterization allows different types of interventions to be performed which can improve postoperative outcomes.
As future strategies, we aim to lower the age of patients in surgery and, where possible, complete the Fontan operation without CPB, so as to reduce effusion time and length of hospital stay.
Conflict of interestsNone declared.
Received 20 July 2011
Accepted 22 November 2011
Corresponding author: Instituto Pediátrico del Corazón, Hospital 12 de Octubre, Avda. de Córdoba s/n, 28041 Madrid, Spain. amendozas.hdoc@salud.madrid.org