Percutaneous aortic valve implantation for patients with severe symptomatic aortic stenosis and a high surgical risk is currently well established. We report our experience in terms of safety and effectiveness of transcatheter aortic valve implantation in other clinical context like the degenerated aortic homografts.
MethodsWe report our initial experience in four hospitals and five patients with degenerated aortic homograft and severe aortic regurgitation, refused for surgery for a heart team, that underwent percutaneous implantation of CoreValve® aortic prosthesis.
ResultsWe included three males and two females. The mean age was 70 (3.5) years. All patients were symptomatic in New York Heart Association class III or IV. Procedures were performed through one of the femoral arteries in all patients and under sedation in three patients. The implant was successfully carried out in all cases. There were no major complications during the procedure or admission and the valvular defect was solved in all cases. In-hospital and 30-days mortality was 0. All patients had clinical improvement during follow-up with a reduction in at less two grades in the New York Heart Association functional scale.
ConclusionsIn our experience the treatment of degenerated aortic homografts and aortic insufficiency with transcatheter aortic valve implantation showed to be safe and effective. The current challenge is to convey the good results of transcatheter aortic valve implantation in symptomatic aortic stenosis and high surgical risk to others disorders of the aortic valve. In the future, it is possible that transcatheter aortic valve implantation will expand its indications to majority of aortic valve disorders and patients with less surgical risk.
Keywords
.
IntroductionSevere aortic stenosis (SAS) is the most prevalent degenerative valve disease in the western world; valve replacement by surgery is the treatment of choice, in view of the extensive experience and good outcomes.1 In the last 10 years, transcatheter aortic valve implantation (TAVI) in inoperable patients, whether because of high surgical risk (logistic EuroSCORE >20%) or due to technical contraindications to surgery, has become increasingly more widespread. The procedure is supported by the good outcomes in registries2 and its simplicity. It has become a recognized alternative for this type of patient,3 and has been shown to be superior to conservative treatment.4 Even in high-risk patients, the PARTNER A study has shown that TAVI is as effective as surgical valve replacement, with no significant differences in 1-year survival, although with a higher incidence of stroke.4 To date, more than 15 000 patients have undergone this type of intervention. Currently, 2 types of transcatheter valves are available, Medtronic CoreValve® System and the Edwards-SAPIEN® System, both of which incorporate a biological prosthesis, but with different supports. For implantation, femoral access has been most widely used but transapical and transaxillary approaches have also been tried with the SAPIEN® and CoreValve®, respectively.5
Encouraged by the good outcomes with these devices implanted in degenerative SAS, a series of publications has appeared recently in which TAVI has been used in other diseases of the aortic valve, with good outcomes, in patients with a high surgical risk. Of note among these are bicuspid aorta6 and aortic bioprosthesis dysfunction, both with stenosis and aortic regurgitation (AR).7 However, in other aortic conditions, experience with this type of prosthesis is very limited, as is the case with homografts (HG). Structural deterioration is the main cause for repeat surgery in these patients for whom surgery is currently the treatment of choice when severe degeneration occurs. The possibility of extending the indication of TAVI to patients with SAS and intermediate surgical risk is under investigation; thus the randomized SURTAVI study is being set up. The aim of that study is to demonstrate that TAVI is not inferior to conventional surgery in these patients. Even so, there are still obstacles to applying the technique to lower risk patients. For example, durability, has not been well determined, and there is a greater incidence of stroke and pacemaker placement compared to conventional surgery. In this line of indications, which we might call off-label use of TAVI, we present the largest published series of treatment of aortic HG dysfunction through transcatheter implantation of a CoreValve® prosthesis.
Methods PatientsFive patients from 4 hospitals in and outside Spain who had dysfunctional aortic HG and a high surgical risk according to the logistic EuroSCORE or technical contraindications for a repeat operation were included. In all cases, patients were selected by a multidisciplinary team made up of clinical and interventional cardiologists and heart surgeons.
ProcedurePrior to the intervention, transthoracic and/or transesophageal echocardiography, coronary angiography, and angiography of the aortic vessels and aortic root, ascending aorta, and aortic value by means of angiography and/or computed tomography (CT) were performed.
All procedures were performed in hospitals with heart surgery capability; they were performed in the cardiac catheterization laboratory, with deep sedation in 4 patients and general anesthesia in 1, and infiltration of local anesthetic at the puncture site. Access was fully percutaneous via the right femoral artery in all cases, with percutaneous closure using the Prostar XL® device. Patients were pretreated with 100mg of acetylsalicylic acid and 75mg of clopidogrel per day. Prior to starting the intervention, antibiotic prophylaxis was administered according to the standard protocol of each hospital for conventional heart surgery, and a temporary pacemaker was implanted in the right ventricle using the jugular or femoral approach. Valve implantation was performed according to standard techniques, as has been described in the literature,8, 9 except for valvuloplasty procedures, which were not necessary in patients with HG dysfunction due to AR. Whether or not transesophageal echocardiography was performed during the procedure was left to the discretion of the investigator.
For the choice of prosthesis size, the diameter of the aortic annulus of the HG was taken into account, according to the summary of product characteristics, although an ad hoc study was performed according to the protocol of each hospital. As a general rule, and according to the specifications for treatment of degenerative SAS, in which there is abundant calcium on the valve, thereby providing an ideal substrate for anchoring the valve, a CoreValve® prosthesis no. 29 was implanted in annuli ≥23 mm and a no. 26 valve if the annulus was <23mm. In cases where there was little calcification and the diameter of the annulus was on the limit (21-22mm), it was left to the judgment of the operator whether to use a prosthesis no. 29 to achieve a more secure placement and better stability after implantation.
DefinitionsSafety: lack of complications derived from the prosthesis or the procedure.
Efficacy: combination of lack of complications and attainment of measurable clinical benefit for the patient.
Implantation success: correct implantation and normal functioning of the prosthesis (assessed by angiography and echocardiography), in the absence of mortality during the procedure.
Complications: According to the criteria established in the consensus statement of the Valve Academic Research Consortium,10 all-cause mortality, cardiovascular mortality, cerebrovascular accident, hemorrhage, acute renal failure, vascular complications, severe AR after implantation, thrombosis or prosthetic endocarditis, need for pacemaker placement, and coronary obstruction after implantation were recorded.
ResultsThe study included 5 patients, 3 men and 2 women, with a mean age of 70 (3.5) years, dysfunctional HG due to severe AR, and dyspnea in an advanced functional class in all cases. The baseline characteristics of these patients are shown in Table 1. The mean logistic EuroSCORE was 20%. Of note is the high prevalence of ischemic heart disease; all but 1 of the patients had a prior aortocoronary bypass and, in addition, 2 patients had more than one prior cardiac intervention. The indication for TAVI was established in 3 patients due to technical contraindications for surgery, and due to high surgical risk in the other 2.
Table 1. Baseline Characteristics of the Patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Age, years | 65 | 69 | 76 | 70 | 74 |
| Sex | Male | Male | Female | Female | Male |
| EuroSCORE log, % | 8.6 | 10.8 | 34.8 | 31.2 | 14.6 |
| NYHA class | IV | III | III | III | III |
| Prior cardiac interventions | 3 | 1 | 1 | 2 | 1 * |
| Aortocoronary bypass | Yes | Yes | Yes | Yes | No |
| HG indication | IPE | AAA | SAS | SAS | SAS and Ao rupture |
| Type of HG surgery | ARR | SC | SC | SC | ARR |
| Age HG, years | 9 | 2 | 12 | 14 | 11 |
| Type of dysfunction | AR | AR | AR | AR | AR |
| LVEF, % | 65 | 50 | 60 | 17 | 41 |
| Aortic annulus diameter, mm | 24 | 26 | 24 | 23 | 22 |
| HG calcification | III/IV | II/IV | I/IV | II/IV | I/IV |
| TEE | Yes | Yes | No | Yes | Yes |
| CT | Yes | Yes | Yes | No | Yes |
AAA, ascending aortic aneurysm; Ao, aorta; AR, aortic regurgitation; ARR, aortic root replacement; CT, computed tomography; EuroSCORE log, European System for Cardiac Operative Risk Evaluation; HG, homograft; IPE, infective prosthetic endocarditis; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SAS, severe aortic stenosis; SC, subcoronary; TEE, transesophageal echocardiography.
* This was one intervention, but it was complicated by bleeding and there were 2 repeat operations during the same admission. For this reason, the patient was not considered for any surgical procedures during follow-up.
Table 2 shows the data pertaining to the procedure and follow-up. Implantation was successful and the valve condition was resolved in all patients, with no complications during the procedure or admission, except for the need for pacemaker placement in one of the patients who had a history of right bundle branch block and first degree atrioventricular block. In one of the patients, prior to release of the prosthesis, withdrawal was performed to repeat the placement, as the position was too low. After the recapture maneuver, the prosthesis was deployed from the releasing catheter and was placed, partially open, in the infrarenal aorta. The valve was dilated at this level to allow the valve to be advanced once again to the aortic valve, and the procedure was brought to a successful conclusion.
Table 2. Characteristics and Outcomes of the Procedure. Mortality and Clinical Follow-up.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Anesthesia | Sedation | Sedation | General | Sedation | Sedation |
| Implantation success | Yes | Yes | Yes | Yes | Yes |
| Number of attempts | 1 | 1 | 1 | 1 | 2 |
| Prosthesis number | 29 | 29 | 29 | 29 | 29 |
| Residual AR | 0 | I | 0 | 0 | II |
| Access | Femoral | Femoral | Femoral | Femoral | Femoral |
| Success of percutaneous closure | Yes | Yes | Yes | Yes | Yes |
| Scope time, min | 18.3 | 24.7 | 19.4 | 11.3 | 22.5 |
| Contrast volume, mL | 315 | 243 | 180 | 270 | 200 |
| Periprocedural TEE | Yes | Yes | Yes | Yes | No |
| VARC complications | No | No | No | No | Yes * |
| Prosthesis migration | No | No | No | No | No |
| In-hospital death | No | No | No | No | No |
| 30-day mortality | No | No | No | No | No |
| Mortality at end of follow-up | No | No | No | No | No |
| Decrease in NYHA ≥2 grades | Yes | Yes | Yes | Yes | Yes |
AR, aortic regurgitation; NYHA, New York Heart Association; TEE, transesophageal echocardiogram; VARC, Valve Academic Research Consortium.
* Definitive pacemaker placement in a patient with right bundle branch block and first degree atrioventricular block in the baseline electrocardiogram.
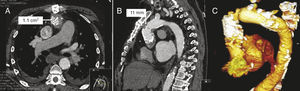
In patient 1, the indication for which the HG had been implanted years before was infective endocarditis; in this case the extreme friability of the tissue of the ascending aorta in the end-to-end anastomosis between the HG and the native aorta, observed during the intervention, required reinforcement with strips of bovine pericardium and biological glue. Over the years, the result was a very restrictive anastomosis that even caused a gradient at this level (Figure 1). In this case, valve implantation had 2 additional difficulties. The first was to cross this small calcified orifice of 11mm with the valve, bearing in mind that the caliber of the deployment sheath was 6mm. The procedure involved an invasive examination to determine whether approach via the femoral artery or the left axillary artery was best. Femoral access was most appropriate, as it was better aligned with the axis of the stenosis. The second difficulty lay in achieving appropriate positioning for the prosthesis, as this had to finish below the stenosis in order to achieve full expansion of the new valve, but at the same time it could not remain deployed very low in the left ventricle, as this would be associated with several AR. It was thus very important to measure the distance between the aortic annulus and the restrictive anastomosis, and several measurements were made in different planes by CT. Bearing in mind that the prosthesis measured 5cm along the long axis and that the annulus-stenosis distance was 5.6cm, the margin of error in its placement was very small. Furthermore, once deployment of the valve had been initiated, the maneuver for recapture in the event of incorrect positioning would be extremely difficult or impossible, because to enable withdrawal, it would be necessary to cross through the small anastomotic orifice with the partially expanded valve. On the other hand, once the prosthesis had been deployed below the stenosis, this helped to keep it in position, preventing distal dislocation (Figure 2).
Figure 1. Computed tomography image (A and B) and 3-dimensional reconstruction (C) of patient 1, with substantial stenosis of the anastomosis between the homograft and the native aorta.
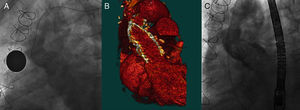
Figure 2. A: Aortic angiography before the procedure. B: Computed tomography reconstruction in 3 dimensions. C: Angiography after implantation of the valve in patient 1.
Patient 5, with a 22mm annulus, received a no. 29 valve, as the limited calcification of the HG did not guarantee appropriate anchoring of prosthesis no. 26; optimal fixation, expansion, and functionality of the prosthesis were achieved and there was no significant transvalvular gradient at the end of the procedure.
After a mean follow-up of 322 (168) days, all patients were still alive and showed substantial clinical improvement and a decrease of at least 2 grades on the functional class from the New York Heart Association scale for dyspnea.
DiscussionAlthough some individual case reports have been published,11 our series is to date the first that reports management of patients with degenerated HG, who in general have a high surgical risk, through transcatheter prosthesis implantation.
Currently, there is a growing group of patients in whom aortic valve and ascending aorta abnormalities coincide. For more than 30 years, the surgical technique of Bentall and De Bono12 has been used in view of its good short- and long-term results. This technique consists of exchanging the aortic root and ascending aorta with a valved tube. The coronary arteries are subsequently reimplanted to this tube. However, another type of intervention is currently gaining acceptance. This consists of using cryopreserved HGs as replacements of the aortic root and/or valve. The technique is particularly indicated in the cases of infective endocarditis on the prosthetic valve or when destruction of the aortic root is occurring.13
Among the possible advantages of this type of intervention is the more physiological behavior or the aortic root in relation to the left ventricle, whereby larger mass regression rates are achieved than with conventional valve replacement.14 Other advantages include the lower incidence of infective endocarditis and thromboembolic disease and no need for anticoagulant treatment.15 Two surgical techniques are available to manage aortic HG, one involves subcoronary implantation and the other aortic root replacement (ARR). Although there are no differences in terms of long-term durability, subcoronary implantation has a greater rate of early incompetence, as we saw in patient 2, in whom dysfunction appeared just 2 years after surgery, whereas ARR has a higher incidence of repeat intervention and periprocedural mortality due to problems involving the reimplantation of coronary arteries, among others.16 ARR also yields better hemodynamic behavior than subcoronary implantation and so is considered the technique of choice.
The structural deterioration of HG is the main cause for repeat operations in these patients. The age of the patient at the time of implantation is one of the elements most strongly associated with this structural deterioration and the need for repeat intervention. This is more frequent the younger the patient, as shown by the now classic study by O’Brien et al.,14 who reported that the rate of repeat intervention at 10 years in patients under 20 years of age was almost 20 times greater than in patients aged over 65 years.
The most frequent form of dysfunction, as in our sample, was calcification and valve regurgitation, which seem to be related to degenerative processes17; currently surgery is the treatment of choice.
Both the EuroScore and STS score, the 2 most widely used risk scales for heart surgery, incorporate repeat intervention as an element indicating higher surgical risk. This risk is higher still in the case of a second or third intervention, although this is only assessed in the STS score. Although the effect of repeat intervention on mortality is always important, mortality is higher the older the patients. Thus, the risk of death at 30 days is 6 times greater with repeat intervention after aortic valve surgery in patients aged over 80 years, and this becomes a major predictor of mortality.18
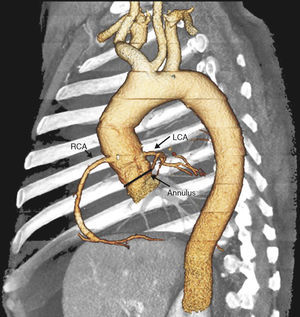
For these reasons, patients with dysfunctional HG have, on one hand, high surgical risk and, on the other, are more likely to have AR. In this context of AR and non-native valve, the indication for TAVI has yet to be established. In our brief initial experience in the treatment of dysfunctional HG, the use of TAVI was safe, was not associated with any important complication, and was effective, as it corrected the valve defect in all cases and the patients achieved clinical benefit. We have identified a series of considerations prior to and during the procedure, other than implantation in the SAS, to adapt the technique to the condition that we wish to treat. Thus, in the prior evaluation of the implant, CT of the aorta can be very valuable for an appropriate evaluation of the HG, with particular assessment of the degree of calcification of the aorta and the implant region. This will help us anchor the prosthesis correctly. It is also important to assess the region of anastomosis between the HG and the ascending aorta which, at times, as we have seen in Patient 1, can be very restrictive. If this is the case, the distance to the annulus should be measured to ensure an appropriate expansion of the valve of the prosthesis. Another aspect to take into account in these patients is the appropriate identification of the origin of both coronary ostia, particularly when the HG has been implanted by the ARR technique. They will usually be in their anatomic site, as the ostia of the HG are used for reimplantation of the coronary arteries, but at times, for technical reasons, these are reimplanted at another site (Figure 3).
Figure 3. Computed tomography reconstruction in 3 dimensions for correct identification of the coronary ostia before the intervention. LCA, left coronary artery; RCA, right coronary artery.
Unlike what has been proposed in TAVI of dysfunctional bioprosthesis,6 for the choice of prosthesis size, the diameter of the annulus measured in the HG before implantation can act as a guide, but an ad hoc measure should be made according to the same protocol as the treatment of the SAS, as degeneration of the HG itself can lead to annulus dilatation. The size of the ascending aorta in these patients, in whom HG degeneration is coupled with its dilatation, could be a limitation when it comes to selecting recipients of a CoreValve®, as its diameter at 5cm from the annulus should be less than 41mm for implantation of valve no. 26 and 43mm for valve numbers 29 or 31. This will be particularly important in patients with a HG without much calcification and, therefore, with a weaker anchoring at the level of the aortic annulus.
With regards to the procedure itself, obviously, in patients with heart failure due to AR, valvuloplasty will not be necessary. An added difficulty when performing the implantation is the stability of the pigtail catheter which is placed in the posterior coronary sinus, marking the position of the annulus and which serves as a reference for prosthesis placement, as well as for measurement of arterial blood pressure and follow-up angiography. In patients with heart failure due to AR, the pigtail is very unstable, which hinders its role of a marker. Other radiological markers should therefore be used, guided by aortography and angiography should be more frequent. In addition, intraprocedural transesophogeal echocardiography should be performed. In principle, we have not found other differences with respect to implantation in degenerative SAS and, as in this case, it is important to study each patient prior to the procedure to design the most appropriate strategy in each case.
ConclusionsThe results obtained in our series indicate that TAVI in dysfunctional HG could be a safe and effective alternative in the short term. These findings need to be confirmed in future registries. Currently, it can be considered that treatment of SAS in patients of high surgical risk with TAVI has now become established in clinical practice, with excellent outcomes in terms of both intrahospital and 30-day mortality and a substantial improvement in the functional class of the patients. Once this initial phase has been overcome, with increasing frequency, this type of therapy is tried in off-label indications, with promising initial results, although with very little accumulated experience to enable firm conclusions to be drawn. New studies are being undertaken in patients with lower risk, in an attempt to extend the indication of TAVI to a large number of aortic valve diseases and to younger patients. To extend the indication to patients of low surgical risk, its superiority compared to conventional surgery has still to be demonstrated in these younger patients. In addition, it is necessary to resolve some of the important limitations such as the higher incidence of the need for pacemakers, the higher incidence of stroke, and the presence of different degrees of residual AR. However, the procedure is a valid option in patients with no other alternative.
Conflict of interestsNone declared.
Received 15 September 2011
Accepted 22 November 2011
Corresponding author: Rua Das Flores 37-A, 15896 Santiago de Compostela, A Coruña, Spain. birihh@yahoo.es