Surgical site infection in cardiac surgery is uncommon. The aim of the present study was to examine the incidence of this infection, compare it with national and international data, and evaluate its risk factors.
MethodsThis prospective cohort study included patients who underwent valve surgery or coronary revascularization during a 6-year period. The incidence of surgical site infection was studied. Associations between risk factors and infection were evaluated using odds ratios (OR). The infection rate was compared with Spanish and American data using the standardized infection ratio.
ResultsA total of 1557 patients were included. The overall cumulative incidence of infection was 4% (95% confidence interval [95%CI], 3.6%-5.6%), 3.6% in valve surgery (95%CI, 2.5%-4.7%) and 4.3% in coronary revascularization (95%CI, 2.3%-6.3%). Risk factors for surgical site infection in valve surgery were diabetes mellitus (OR=2.8; P<.05) and obesity (OR=6.6; P<.05). Risk factors for surgical site infection in coronary revascularization were diabetes mellitus (OR=2.9; P<.05) and reoperation for bleeding (OR=8.8; P<.05).
ConclusionsDiabetes mellitus and obesity favor surgical site infection in valve surgery, whereas diabetes mellitus and reoperation for bleeding favor surgical site infection in coronary revascularization. Infection surveillance and control programs permit evaluation and comparison of infection rates in cardiac surgery.
Keywords
Surgical site infection (SSI) is an uncommon complication of cardiac surgery associated with high morbidity and mortality. It increases health care costs, mean hospital length of stay, and the rates of reoperation and intensive care unit admission.1–4 The profile of patients undergoing cardiac surgery has changed in recent years, with greater operative complexity (multiple valve or mixed valve and coronary artery surgeries), fewer patients undergoing isolated coronary revascularization, and more comorbidities.5,6
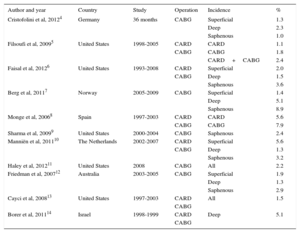
The incidence of SSI in cardiac surgery varies between 1.1% and 7.9% according to the surgical procedure analyzed (Table 1). Because this variation is due to differences in study design, patient profile, type of procedure analyzed, and the definitions used for infection classification, implementation of infection surveillance and control programs in hospitals is important. Such a program would permit evaluation of the incidence of infections and related risk factors, assessment of changes in epidemiological patterns, comparisons with other centers, and on-going determination of the effectiveness of any preventive measures introduced.7–14
Studies of the Incidence of Surgical Site Infection after Cardiovascular Surgery
| Author and year | Country | Study | Operation | Incidence | % |
|---|---|---|---|---|---|
| Cristofolini et al, 20124 | Germany | 36 months | CABG | Superficial | 1.3 |
| Deep | 2.3 | ||||
| Saphenous | 1.0 | ||||
| Filsoufi et al, 20095 | United States | 1998-2005 | CARD | CARD | 1.1 |
| CABG | CABG | 1.8 | |||
| CARD+CABG | 2.4 | ||||
| Faisal et al, 20126 | United States | 1993-2008 | CARD | Superficial | 2.0 |
| CABG | Deep | 1.5 | |||
| Saphenous | 3.6 | ||||
| Berg et al, 20117 | Norway | 2005-2009 | CABG | Superficial | 1.4 |
| Deep | 5.1 | ||||
| Saphenous | 8.9 | ||||
| Monge et al, 20068 | Spain | 1997-2003 | CARD | CARD | 5.6 |
| CABG | CABG | 7.9 | |||
| Sharma et al, 20099 | United States | 2000-2004 | CABG | Saphenous | 2.4 |
| Manniën et al, 201110 | The Netherlands | 2002-2007 | CARD | Superficial | 5.6 |
| CABG | Deep | 1.3 | |||
| Saphenous | 3.2 | ||||
| Haley et al, 201211 | United States | 2008 | CABG | All | 2.2 |
| Friedman et al, 200712 | Australia | 2003-2005 | CABG | Superficial | 1.9 |
| Deep | 1.3 | ||||
| Saphenous | 2.9 | ||||
| Cayci et al, 200813 | United States | 1997-2003 | CARD | All | 1.5 |
| CABG | |||||
| Borer et al, 201114 | Israel | 1998-1999 | CARD | Deep | 5.1 |
| CABG |
CABG, coronary artery bypass grafting; CARD, valve and cardiac structure surgery.
The National Nosocomial Infections Surveillance Index (NNIS) risk index is used to classify patients according to their risk of SSI and compare the adjusted rates among different surgeons, units, centers, and countries. The NNIS combines the degree of operative contamination (the factor most associated with SSI), the American Society of Anesthesiologists (ASA) physical status score (the intrinsic risk of the patient), and the operative time (an indicator of the complexity of the surgical procedure).15 Patients undergoing cardiac surgery are fairly homogeneous with respect to these characteristics. Because most cardiac procedures are clean operations and the ASA score is usually > II, the NNIS is only able to categorize patients into 2 large groups according to the operative time: high risk of SSI with long operative times and low risk with shorter durations.
Any differences in the distribution of some of the associated variables revealed by a comparison of SSI incidences might be due to a confounder. Thus, to enable the intercomparison of populations with different structures, they can be homogenized through the use of an epidemiological method called standardization. This approach requires knowledge of the SSI distribution according to the NNIS risk of the populations being compared and the specific rates of the reference population according to the NNIS risk index. This method is very useful when comparing small populations with few cases in one or more subgroups.16
On the other hand, although the NNIS is an internationally accepted method for the comparison of SSI rates, the avoidable causes and/or risk factors related to the SSI need to be identified in order to design and implement prevention and control strategies. The objective of our study was to determine the incidence of SSI in cardiac surgery, adjust and compare our incidence with published data from inside and outside Spain, and evaluate the risk factors related to SSI to identify those with greater risk of influencing SSI.
METHODSThis prospective cohort study was performed in the Hospital Universitario de La Princesa, Madrid, Spain, a tertiary care center with a reference population of about 500 000 inhabitants.
The study included all patients who underwent cardiac surgery between January 1st 2009 and December 31st 2014. The included patients underwent valve surgery (cardiac surgery) and/or chest only or chest and donor site coronary revascularization (coronary artery bypass surgery). The following patients were excluded: those receiving antibiotic therapy, those with a diagnosis of infection at the time of surgery, and those aged <18 years.
Sample size estimation was performed according to an expected SSI incidence of 3%, 95% confidence interval (95%CI), margin of error of 1%, and loss of 1%. An estimated sample size of 1129 patients was required. The SSI criteria of the Centers for Disease Control and Prevention (CDC) were used and the depth of the infection was classified as superficial, deep, and organ-space.1 The study was approved by the Ethics Committee and Research Commission of the Hospital Universitario de La Princesa.
Daily follow-up was performed in all patients included in the study from admission to hospital discharge by staff trained in infection surveillance and control. A record was made of all readmissions related to a complication and/or infection within 1 year after the surgery. This cutoff was chosen because it is the maximum incubation period of an infection of an implant surgery-related surgical wound according to the surveillance criteria of the CDC.1
Data were gathered from the clinical history, nursing records, surgical reports, microbiological cultures, and information provided by the physicians and nurses providing health care for the patients. We collected information on clinicoepidemiological variables (age, sex, diabetes mellitus [DM], chronic obstructive lung disease, chronic renal failure, obesity) and admission-related variables (type of admission, diagnosis, total stay, length of preoperative and postoperative stay, length of intensive care unit stay, type of discharge, readmission for complications), operative variables (type of surgery, procedure, degree of contamination, operative time, ASA score, reoperation, preoperative preparation, perioperative prophylaxis), and infection variables (type of infection, location, date, and culture).
The Clinical Indicators of Continuous Quality Improvement (INCLIMECC) program17 program was used as a working tool because this epidemiological surveillance system is used by about 100 Spanish hospitals for health care-related infections. This program uses standardized data collection protocols, the diagnostic criteria for infections proposed by the CDC, and the International Classification of Diseases (Ninth Revision, Clinical Modification) for coding patients’ diagnoses and procedures.
Surgical site infections were defined as infections related to the surgical procedure developing in the surgical incision or its vicinity during the first 30 postoperative days or within 1 year if a prosthesis had been implanted. A prosthesis was defined as any nonhuman object, material, or tissue permanently implanted during a procedure that was ordinarily not manipulated for diagnostic or therapeutic purposes.1,12,18
The preoperative protocol for patient preparation consisted of a bath with antiseptic soap, rinse with antiseptic mouthwash, and, if necessary, an electric shave. The surgical field was prepared with topical antiseptic solution. Sternotomy closure was performed using steel wire for the sternum while soft tissue was closed using a layered technique with sterile synthetic absorbable suture material. Topical antiseptic solution was applied at the edges of the skin, which was closed with surgical staples. Antibiotic prophylaxis was performed with cefazolin 2g at anesthesia induction and 1g/8h for 48hours (or vancomycin for patients allergic to beta-lactams). The classification proposed by the National Research Council was used to classify the degree of bacterial contamination in the surgical field (clean, clean-contaminated, contaminated, and dirty).
The NNIS risk index stratified the patients into 4 levels of SSI risk according to the presence of the following factors: a) degree of surgical contamination (this factor was considered present if the operation was contaminated or dirty); b) ASA score of anesthetic risk (this factor was considered present if the ASA value was > II), and c) operative duration (this factor was considered present if the operative time was greater than the 55th percentile (p75) of the procedure analyzed).16,18
Statistical AnalysisTo compare our SSI rates, we used the cumulative data of the INCLIMECC-Spain network (1997-2012)17 and the latest data (2006-2008) published by the National Healthcare Safety Network (NHSN) of the United States18 because both systems use the same diagnostic, classification, and stratification criteria. Because any comparison of the crude rates might not be adequate unless the population structures are comparable, indirect standardization was performed using the NNIS risk index as an adjustment factor. The product of the SSIs observed in the study and the specific rates according to the NNIS risk index of the reference population provided the number of expected SSIs if the study population were to show standard rates. Division of the total number of cases observed by the total number of expected cases provided the standardized infection ratio (SIR), which is methodologically interpreted as a relative risk.19
For the descriptive analysis of qualitative variables, frequencies were calculated; for their comparison, the Pearson chi-square test or Fisher's nonparametric exact test was used. The mean ± standard deviation was calculated for quantitative variables. Their comparison was performed with the Student t test or the nonparametric Mann-Whitney U test.
An explanatory model of SSI was constructed using multivariable logistic regression analysis, derived from a saturated model containing all variables with a statistically significant association in the univariate analysis and those considered related to SSI development with no significant association. A backward stepwise exclusion strategy was used to obtain the final model, evaluating the goodness of fit with the Hosmer-Lemeshow test and calculating the corresponding odds ratios (ORs). Data analysis was performed with the INCLIMECC program and SPSS statistical software version 19.0 for Windows. Differences were considered statistically significant at P<.05.
RESULTSDuring the study period, 1557 suitable patients were admitted to the cardiovascular surgery service. In these patients, 1666 surgical procedures were analyzed, with a ratio of 97 major surgical procedures per year per surgeon: 67% were valve operations (1119 procedures), 17% were coronary artery bypass graft operations (281), 9% were mixed coronary artery/valve surgeries (157), and 6.5% were reoperations for complications (109).
The mean age of the patients was 70±12 years and 58% of the patients were men. Of the comorbidities analyzed, the risk factor most frequently associated with cardiac surgery was DM, present in 27% of the interventions. The mean length of stay was 21±19 days, 6±6 days for the preoperative stay and 16±17 days for the postoperative stay. Patient characteristics and differences between infected and noninfected patients are shown in Table 2.
Characteristics of the Study Population
| All (n=1557) | Without infection (n=1485) | With infection (n=72) | P | |
|---|---|---|---|---|
| Age, y | 70±12 | 70±12 | 71±11 | > .05 |
| Men, % | 58 | 58 | 51 | > .05 |
| Diabetes mellitus, % | 27 | 25 | 50 | <.05 |
| CRF, % | 8 | 8 | 6 | > .05 |
| COPD, % | 4 | 4 | 7 | > .05 |
| Obesity, % | 5 | 4 | 17 | <.05 |
| Mean stay, d | 21±19 | 21±19 | 23±69 | > .05 |
| Preoperative stay, d | 6±6 | 6±6 | 5±4 | > .05 |
| Postoperative stay, d | 16±79 | 16±17 | 18±16 | > .05 |
| ICU stay, d | 4±8 | 4±8 | 4±5 | > .05 |
COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; ICU, intensive care unit.
Unless otherwise indicated, values are expressed as mean±standard deviation.
Of the surgical procedures performed, 95% were elective, 93% were clean operations, and 88% of the patients had ASA scores > II. The total operative time was 276±81min, with 30% of operations exceeding the p75 (Table 3). There were 87 reoperations for bleeding (5%) and 22 SSIs (1.3%).
Characteristics of the Surgical Procedures Analyzed
| All, % (n=1557) | Without infection, % (n=1485) | With infection, % (n=72) | P | |
|---|---|---|---|---|
| Elective surgery | 95 | 95 | 94 | > .05 |
| Surgical procedure | < .05 | |||
| CARD | 72 | 73 | 56 | |
| CABG | 18 | 17 | 32 | |
| Mixed | 10 | 10 | 13 | |
| Clean surgery | 92 | 93 | 94 | > .05 |
| ASA score ≥ III | 88 | 88 | 94 | > .05 |
| Operative time ≥ p75* | 30 | 30 | 47 | < .05 |
| Reoperation for bleeding | 5.6 | 5.1 | 16.7 | < .05 |
| Adequate prophylaxis | 98 | 98 | 97 | > .05 |
| Adequate preparation | 93 | 93 | 87 | > .05 |
ASA, American Society of Anesthesiologists; CABG, coronary artery bypass grafting; CARD, valve surgery; p75, 75th percentile.
*Percentile of valve surgery (330 min) and coronary artery bypass grafting (340 min).
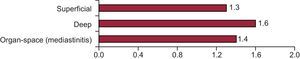
The overall incidence of SSI was 4% (95%CI, 3.6%-5.6%). The incidence of infection according to depth is shown in Figure 1.
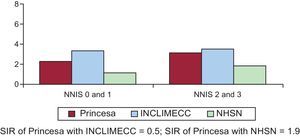
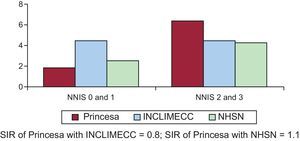
The incidence of SSI in the 1119 valve operations analyzed was 3.6% (95%CI, 2.5%-4.7%). The SIRs obtained by the comparison of our incidence with that of national data and the latest data published by the NHSN were 0.5 (95%CI, 0.2-0.9) and 1.9 (95%CI, 1.2-2.5), respectively (Figure 2). At least one of the mammary arteries was used in 95.7% of the 281 coronary artery bypass graft surgeries analyzed. In addition, the incidence of primary SSI was 4.3% (95%CI, 2.3%-6.3%) and the incidence of secondary SSI (incision for saphenous vein graft extraction) was 3.0% (95%CI, 0.0%-5.2%). In coronary revascularization, a distinction was made between coronary artery bypass with chest and donor site incisions (217 procedures) and single chest incision (64 procedures). The incidences of SSI were 3.6% (95%CI, 1.7%-5.5%) and 7.6% (95%CI, 1.8%-13.4%), respectively. Upon comparison of our series with data published by the INCLIMECC network, the SIR was 0.8 (95%CI, 0.1-1.7) in coronary revascularization with chest and donor site incisions and 3.0 (95%, 0.8-6.8) in coronary revascularization with chest incision only, with no statistically significant difference between them. The same occurred upon comparison of our results with those of the NHSN, with SIRs of 1.1 (95%CI, 0.1-2.2) for coronary revascularization with chest and donor site incisions and 4.5 (95%CI, 0.1-9.0) for single chest incision (Figures 3 and 4).
Incidence of surgical site infection after valve surgery according to the National Nosocomial Infections Surveillance Index. INCLIMECC, Clinical Indicators of Continuous Quality Improvement; NHSN, National Healthcare Safety Network; NNIS, National Nosocomial Infections Surveillance Index; SIR, standardized infection ratio.
Incidence of surgical site infection after coronary revascularization with chest and donor site incisions according to the National Nosocomial Infections Surveillance Index. INCLIMECC, Clinical Indicators of Continuous Quality Improvement; NHSN, National Healthcare Safety Network; NNIS, National Nosocomial Infections Surveillance Index; SIR, standardized infection ratio.
Incidence of surgical site infection after coronary revascularization with chest incision only according to the National Nosocomial Infections Surveillance Index. INCLIMECC, Clinical Indicators of Continuous Quality Improvement; NHSN, National Healthcare Safety Network; NNIS, National Nosocomial Infections Surveillance Index; SIR, standardized infection ratio.
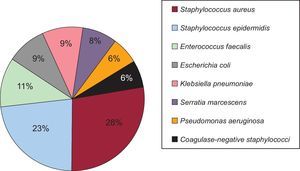
The causative microorganism could not be identified in 92% of the SSIs. Of the 78 microorganisms cultured, the most frequently isolated were Staphylococcus aureus (28%), Staphylococcus epidermidis (23%), Enterococcus faecalis (11%), Escherichia coli (9%), and Klebsiella pneumoniae (9%) (Figure 5). Of the SSIs, 38% were polymicrobial.
Univariate analysis of the association between the SSI and the risk factors revealed significant differences according to the presence of DM (OR=1.1; 95%CI, 1.03-1.10), obesity (OR=1.1; 95%CI, 1.02-1.26), surgical procedure (OR=1.1; 95%CI, 1.01-1.09), operative time > p75 (OR=1.3; 95%CI, 1.07-1.66), and reoperation for bleeding (OR=1.1; 95%CI, 1.02-1.21). Multivariable analysis confirmed the following variables to be independent risk factors for SSI: DM (OR=3.0; 95%CI, 1.8-4.8), obesity (OR=4.0; 95%CI, 2.0-8.0), operative time (OR=1.0; 95%CI, 1.00-1.01), and reoperation for bleeding (OR=4.0; 95%CI, 1.9-7.6).
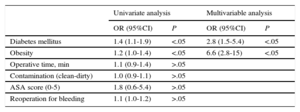
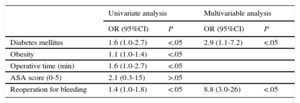
The risk factors associated with SSI development according to the type of surgical procedure, identified using logistic regression, are presented in Table 4 (valve surgery) and Table 5 (coronary artery bypass grafting).
Association Between Risk Factors and Surgical Site Infection in Valve and Cardiac Structure Surgery (n=1119)
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Diabetes mellitus | 1.4 (1.1-1.9) | <.05 | 2.8 (1.5-5.4) | <.05 |
| Obesity | 1.2 (1.0-1.4) | <.05 | 6.6 (2.8-15) | <.05 |
| Operative time, min | 1.1 (0.9-1.4) | >.05 | ||
| Contamination (clean-dirty) | 1.0 (0.9-1.1) | >.05 | ||
| ASA score (0-5) | 1.8 (0.6-5.4) | >.05 | ||
| Reoperation for bleeding | 1.1 (1.0-1.2) | >.05 | ||
95%CI, 95% confidence interval; ASA, American Society of Anesthesiologists; OR, odds ratio.
Association Between Risk Factors and Coronary Artery Bypass Grafting Infection (n=281)
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Diabetes mellitus | 1.6 (1.0-2.7) | <.05 | 2.9 (1.1-7.2) | <.05 |
| Obesity | 1.1 (1.0-1.4) | <.05 | ||
| Operative time (min) | 1.6 (1.0-2.7) | <.05 | ||
| ASA score (0-5) | 2.1 (0.3-15) | >.05 | ||
| Reoperation for bleeding | 1.4 (1.0-1.8) | <.05 | 8.8 (3.0-26) | <.05 |
95%CI, 95% confidence interval; ASA, American Society of Anesthesiologists; OR, odds ratio.
The SSI prevention measures evaluated were perioperative prophylaxis and preoperative patient preparation. In 96% of the cases, the perioperative antibiotic prophylaxis protocol was adapted to the indication, choice of antibiotic, time of initiation, route of administration, and duration. Analysis of the causes of inadequate therapy revealed that 48% were due to an extended duration of prophylaxis, 26% to the antimicrobial choice, 13% to the indication, and another 13% to more than one of these causes. Inadequacy was not found to be due to the time of prophylaxis initiation or the route of antibiotic administration. A total of 93% of the patients were prepared in accordance with the protocol in place in the hospital. The preoperative preparation was unknown in the remaining 7% because the clinical history was missing this information. No differences were found between valve surgery and coronary revascularization (P>.05).
DISCUSSIONThe incidence of SSI observed in our study is similar to that published by national and international surveillance systems using the same methodology and the same diagnostic and classification criteria as our surveillance system.18,19
After standardization of the results to compare our data with those published by the INCLIMECC-Spain group and the NHSN of the United States, our incidence of SSI after valve surgery was 50% lower than in the other Spanish hospitals comprising the surveillance network and double that of recent years published by the NHSN. We are unable to rule out the possibility that the SIR values obtained in coronary revascularization are due to chance, possibly because of the small number of procedures analyzed.
In our series, the risk factors independently associated with the development of SSI after cardiac surgery were DM, obesity, operative time, and reoperation for bleeding. The following variables classically associated with SSI were not included in the explanatory logistic regression model: age, chronic renal failure, chronic obstructive pulmonary disease, preoperative stay, degree of operative contamination, and ASA score. Our results agree with those of other authors who have studied the risk factors for SSI in cardiac surgery, principally after coronary revascularization. Haley et al11 observed that inclusion of DM, obesity, renal failure, chronic obstructive pulmonary disease, sex, and reoperation for bleeding significantly improved their ability to predict SSI in their patients. Friedman et al12 and Chen et al20 found DM and body mass index to be the main factors associated with SSI development after coronary revascularization.
A major group of patients at risk of SSI is the elderly.21 In our series, there was no association between age and infection development, and the mean age of our patients was 70 years. These results are in accordance with those published by Filsoufi et al,5 Haley et al,11 Cayci et al,13 and Borer et al,14 who also found no association between age and SSI after cardiovascular surgery. This lack of an association may be because, in this kind of surgical procedure, age is a confounding factor due to its high associated morbidity, and is not a true risk factor. In our patients, DM was a risk factor, an association shown in many other studies.10,16,22,23 Obesity is another risk factor for SSI, due to a decreased blood flow in the adipose tissue and its consequent effect on the scarring process.3 In our patients, obesity was an independent risk factor for infection, as in other series.11,22,23 In the group of patients who underwent coronary revascularization, chronic obstructive pulmonary disease was not a risk factor for SSI, in contrast to the series of Vogel et al,3 Filsoufi et al,5 and Haley et al.11 Extended preoperative stay also increases the risk of SSI.4,5,24,25 The reason for this association is unknown, but it is believed to be related to an increase in the endogenous microorganism reservoir of the patient, the acquisition of hospital flora, or the proliferation of the patients’ own flora. In our series, the preoperative stay was long.
The coding of the elective surgery also included patients with previous stays in other departments such as internal medicine and cardiology, where they were admitted for decompensated heart disease, infectious endocarditis, or de novo studies. These patients underwent operations during their admission, extending their preoperative stay. Analysis of the impact of preoperative stay on infection development failed to reveal significant differences between the group of patients who developed SSI (5.8±6.1 days) and those who did not (4.9±3.7 days). Neither were differences seen when the patients were analyzed according to the type of problem: valve, 5.7±6.4 days with SSI vs 5.3±3.8 days without SSI; coronary artery bypass grafting, 5.9±5.4 days with SSI vs 5.1±3.8 days without SSI (P>.05).
The mean operative time in the study was 276±77minutes. Univariate analysis revealed an association between operative time and SSI development because patients with times>p75 had a 1.3 times higher risk of infection development. After controlling for other covariables in the multivariable analysis, this relationship was not maintained in valve surgery or coronary revascularization, in agreement with other studies.26,27
Comparison of our results with other indices before the start of the study period (2007-2008) revealed no decrease in the incidence of SSI. However, adherence to the protocol increased in terms of antibiotic prophylaxis (88% vs 96%; P<.05) and preoperative patient preparation (55% vs 93%; P<.05).
LimitationsPossible limitations of our study include the nonexhaustive follow-up of patients after discharge. To minimize the possible underestimation of the incidence of SSI, we reviewed all readmissions of patients for 1 postoperative year. We believe that there were no significant losses because most SSIs after cardiac surgery have sufficient impact to require hospital readmission, and the CDC particularly recommends follow-up after discharge for laparoscopic procedures, due to the short hospital stay.1,15,16,18 According to Llanos et al28 and Huenger et al,29 the incidence of SSI after cardiac surgery is not significantly altered by the inclusion of infections developing after discharge that do not require readmission, and the resources required to perform this type of follow-up would overload the surveillance and control system. Because we did not perform follow-up after discharge, we were unable to analyze the competing risk of death (patients without the possibility of SSI development due to their death) although, given that the mortality in the cardiovascular surgery unit during the study period was 5.2%, we estimate that it would not greatly affect the incidence of SSI in our series. Another limitation might be the omission of some variables previously associated with SSI. Other noninfection-related complications were also omitted, such as noninfectious sternal deshiscence.30 The most frequent risk factors were included, but several risk factors described in various studies or that are currently being discussed were not considered: concurrent infections, immune suppression, nutritional status, intraoperative blood loss or blood transfusion requirement, and previous sternotomy or mastectomy.
The risk factors identified (DM, obesity, operative time, surgical procedure, and reoperation for bleeding) are not easily controlled. The operative time can be long for more complex surgical procedures and more detailed analysis of this variable is required because it is not in itself a cause of infection. The identification of obesity as a risk factor for SSI suggests the value of weight loss programs for all patients indicated for elective valve surgery. Because DM was a clear risk factor for all types of cardiac surgery (valve and myocardial revascularization) in our series, protocols should be designed and implemented to control blood glucose in the perioperative period.
Structured and standardized epidemiological surveillance is the basis of all infection control programs. The INCLIMECC program is a prospective system for the surveillance of hospital infection that uses standardized data collection protocols based on the NNIS system and the infection diagnostic criteria proposed by the CDC. Thus, the resulting indicators not only allow comparisons among hospitals participating in the network, but also among other centers and countries following a similar methodology. One of the key points of any surveillance and control system of health care-related infections is to allow comparison of results. The best comparison methodology for the incidence of SSI is the SIR,15 whose purpose is to avoid confounding phenomena due to differences in the distribution of factors related to the infection of the populations being compared.
CONCLUSIONSObesity and DM are risk factors for SSI in valve surgery, whereas, in coronary revascularization, the risk factors were DM and reoperation for bleeding.
Our infection surveillance and control system allows us to evaluate and compare the rates of infection in cardiac surgery with those of national and international reports, revealing similar SSI rates.
All health centers should have infection surveillance and control programs that are able to quantify the frequencies of infections and determine patients’ risk factors, as well as to enable comparison of the results with those of other centers and evaluation of the effectiveness of the preventive and control measures established.
CONFLICTS OF INTERESTNone.
- Surgical infection is an uncommon complication of cardiac surgery associated with high morbidity and mortality. Numerous variables have been linked to infection, such as advanced age, peripheral artery disease, obesity, DM, chronic obstructive pulmonary disease, chronic renal disease, operative time, perioperative bleeding, antibiotic prophylaxis, and preoperative preparation. It is important to determine the clinicoepidemiological characteristics of these patients in our population to design better infection prevention and control strategies.
WHAT DOES THIS STUDY ADD?- We analyzed the factors associated with surgical infection in coronary revascularization and valve surgery, a topic little studied in Spain. We calculated the incidence of infection and compared it with that reported both inside and outside of Spain with an indirect standardization of rates using the NNIS risk index of infection as an adjustment factor. We found a similar incidence to that previously reported and identified specific risk factors for each type of procedure (DM, obesity, and reoperation for bleeding). Preventive measures and perioperative control of these factors could reduce the incidence of infection.