The percutaneous mitral valve repair procedure (MitraClip) appears to reduce mitral annulus diameter in patients with functional mitral regurgitation, but the relationship between this and regurgitation severity has not been demonstrated. The aim of this study was to determine the effect of mitral annulus remodeling on the reduction of mitral regurgitation in patients with functional etiology.
MethodsThe study included all patients with functional mitral regurgitation treated with MitraClip at our hospital until January 2015. Echocardiogram (iE33 model, Philips) was performed in all patients immediately after device positioning. Changes in the mitral annulus correlated with mitral regurgitation severity, as assessed using the effective regurgitant orifice area.
ResultsThe study included 23 patients (age, 65±14 years; 74% men; left ventricular ejection fraction, 31%±13%; systolic pulmonary artery pressure, 47±10 mmHg). After the procedure, the regurgitant orifice area decreased by 0.30 cm2±0.04 cm2 (P<.0005), from a baseline of 0.49 cm2±0.09 cm2. Anteroposterior diameter decreased by 3.14 mm±1.01 mm (P<.0005) from a baseline of 28.27 mm±4.9 mm, with no changes in the intercommissural diameter (0.50 mm±0.91 mm vs 40.68 mm±4.7 mm; P=.26). A significant association was seen between anteroposterior diameter reduction and regurgitant orifice area reduction (r=.49; P=.020).
ConclusionsIn patients with functional mitral regurgitation, the MitraClip device produces an immediate reduction in the anteroposterior diameter. This remodeling may be related to the reduction in mitral regurgitation.
Keywords
Moderate to severe functional mitral regurgitation (MR) has a poor long-term prognosis.1–4 However, currently there is no effective surgical treatment for this condition; a surgical approach is indicated only in patients with moderate to severe MR who are scheduled to undergo coronary revascularization or another type of cardiac procedure.5,6 A recently published study showed no differences in 1-year morbidity and mortality between bypass surgery plus repair of moderate mitral valve disease and revascularization alone.7
The percutaneous mitral repair procedure (MitraClip) is a new percutaneous implantation technique for the treatment of some causes of MR and is based on the Alfieri surgical technique.8,9 However, some studies have demonstrated worse outcomes of this surgical technique when it is not performed with annuloplasty.10 This suggests that the percutaneous technique could be less advantageous than the Alfieri surgical technique because the percutaneous technique does not involve annuloplasty to reduce the annulus size.
Other authors have reported that in functional MR, mitral annulus remodeling occurs after device positioning.11 However, as far as we know, no study has demonstrated a correlation between a reduction in mitral annulus size following percutaneous mitral valve repair and improved reduction in MR immediately after device release.
Therefore, the aim of this study was to analyze the changes in mitral annulus geometry produced immediately after percutaneous device implantation, and to analyze if this potential remodeling is associated with the reduction in regurgitation in functional MR.
METHODSThis was a prospective study of all patients with functional MR treated with Mitraclip in our hospital. All patients underwent a complete echocardiographic study using 2-dimensional and 3-dimensional (3D) transthoracic and transesophageal echocardiography during the procedure, immediately before and after device implantation (iE 33 model, software QLAB v7.0, Philips; Amsterdam, The Netherlands).
Mitral regurgitation severity was assessed according to the criteria from current European guidelines,5 and stratified into 4 grades according to the criteria from the EVEREST trial,8,9 with assessment of the effective regurgitant orifice area (EROA) as the semiquantitative method of choice; mitral regurgitation was considered significant12 at>0.20cm2.
The procedure was considered successful when mitral regurgitation evaluated with transesophageal echocardiography immediately after device release was ≤ grade II.
For the acquisition of 3D images, an X7-2t transesophageal probe was used. Particular care was taken to ensure comparable hemodynamic conditions (blood pressure and heart rate) before and after implantation when assessing mitral regurgitation: fluids and pharmacotherapy were used when necessary.
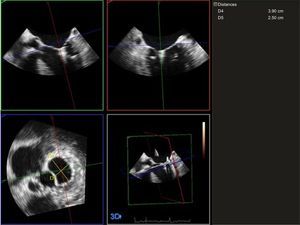
Regarding the study of the mitral annulus, an experienced echocardiographer acquired images using a magnified 3D sequence (Zoom 3D, Philips), after optimizing the image size, the gain, the dynamic range, and other echocardiographic settings, and with electrocardiographic monitoring guidance. Single-beat images were obtained, with a minimum of 9 volumes per second. At least 3 sequences were obtained for patients who were in sinus rhythm and at least 5 sequences for those who were in atrial fibrillation. These images were exported to a workstation equipped with mitral valve analysis software (QLAB v7.0; Bothell, United States). A 3D model was then reconstructed of the mitral valve at end-systole. After optimal alignment in the 3 spatial planes, a long axis was obtained that was taken to be the intercommissural diameter, as well as a short axis that was taken to be the anteroposterior diameter13,14 (Figure 1). The final data obtained were an average of the different sequences acquired.
Three-dimensional measurements of the intercommissural (D4) and anteroposterior (D5) diameters of the mitral annulus after percutaneous device implantation. These measurements were performed by image analysis at end-systole, after optimization of the different echocardiographic settings and after adequate alignment of the annulus in the 3 spatial planes.
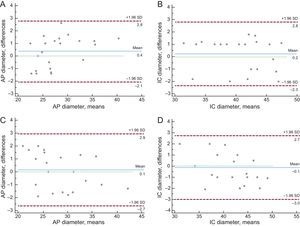
Intraobserver and interobserver variability were also analyzed, for both the anteroposterior annulus diameter and the intercommissural diameter before percutaneous device implantation, in all patients included in the study (Figure 2).
Assessment of the postimplant EROA was based on the sum of the EROAs of the 2 regurgitant orifices.15
If more than 1 device was implanted in a patient, analysis of the mitral annulus and the degree of mitral regurgitation were performed after the release of the second device. Given that in these patients the second clip is usually placed beside the first (at least in our series), regurgitation between the 2 devices is usually minimal. Therefore, it does not create a measureable area of significant isovelocity. With these patients, we analyzed the regurgitation jets lateral and medial to the devices, and used the sum of the jets, as was done in the other patients.
This study was carried out in line with the principles of the Declaration of Helsinki. It was approved by the local ethics committee and the participants gave their informed consent to participate.
Regarding the statistical analysis, qualitative variables are expressed as absolute number and percentage, and quantitative variables are expressed as mean±standard deviation. The Student t test for paired data was used to analyze the relationship between variables. The Pearson correlation coefficient was used for analysis of the correlation between the reduction in the anteroposterior and intercommissural diameters and the reduction in MR severity evaluated using the EROA: this was the primary goal of this study. To confirm the consistency of the findings, the correlation between the percentage reduction in the diameters and the reduction in MR severity was investigated. In addition, the calculations were repeated excluding extreme values. Finally, the coefficients were calculated from a simple linear regression model of the change in the regurgitant orifice area compared against the change in the anteroposterior diameter. Bland-Altman plots and the intraclass correlation coefficient were used for the analysis of intraobserver and interobserver variability in all patients. In all cases, a P-value<.05 was considered statistically significant. The software program PASW v.18 was used.
RESULTSFrom October 2012 (when the Mitraclip was first implanted in our hospital) until January 2015, 29 patients with severe MR were treated using the percutaneous technique; 23 of these patients had functional MR, making up the majority of patients in this study.
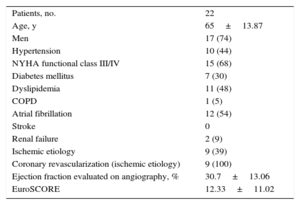
The clinical characteristics of the patients assessed are shown in Table 1. The mean age was 65 years±13.87 years and 74% were male. A 68% were in New York Heart Association functional class III/IV. The mean logistic EuroSCORE was 12.33±11.02, and 79% of the patients had associated pulmonary hypertension. Immediate implant success was achieved in 22 of the patients treated (95.6%). In 1 patient, in the final echocardiographic assessment, release of the posterior leaflet was observed after withdrawal of all catheters; therefore a second clip was implanted during the same procedure. Given the potential effect on annulus geometry from a partially-caught clip, it was decided to exclude this patient from the study sample.
Baseline Clinical Characteristics
| Patients, no. | 22 |
| Age, y | 65±13.87 |
| Men | 17 (74) |
| Hypertension | 10 (44) |
| NYHA functional class III/IV | 15 (68) |
| Diabetes mellitus | 7 (30) |
| Dyslipidemia | 11 (48) |
| COPD | 1 (5) |
| Atrial fibrillation | 12 (54) |
| Stroke | 0 |
| Renal failure | 2 (9) |
| Ischemic etiology | 9 (39) |
| Coronary revascularization (ischemic etiology) | 9 (100) |
| Ejection fraction evaluated on angiography, % | 30.7±13.06 |
| EuroSCORE | 12.33±11.02 |
COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
Hypertension: regular figure > 140/90mmHg or on antihypertensive treatment; dyslipidemia: total cholesterol > 200 mg/dL, and/or triglycerides > 150 mg/dL, and/or lipid-lowering therapy.
Values are expressed as no. (%) or mean±standard deviation.
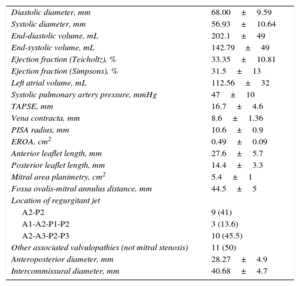
The other echocardiographic parameters are shown in Table 2. Notably, this sample group had significant left ventricular systolic dysfunction and dilatation, with quantitative data of severe mitral regurgitation.
Baseline Echocardiographic Parameters (n=22)
| Diastolic diameter, mm | 68.00±9.59 |
| Systolic diameter, mm | 56.93±10.64 |
| End-diastolic volume, mL | 202.1±49 |
| End-systolic volume, mL | 142.79±49 |
| Ejection fraction (Teicholtz), % | 33.35±10.81 |
| Ejection fraction (Simpsons), % | 31.5±13 |
| Left atrial volume, mL | 112.56±32 |
| Systolic pulmonary artery pressure, mmHg | 47±10 |
| TAPSE, mm | 16.7±4.6 |
| Vena contracta, mm | 8.6±1.36 |
| PISA radius, mm | 10.6±0.9 |
| EROA, cm2 | 0.49±0.09 |
| Anterior leaflet length, mm | 27.6±5.7 |
| Posterior leaflet length, mm | 14.4±3.3 |
| Mitral area planimetry, cm2 | 5.4±1 |
| Fossa ovalis-mitral annulus distance, mm | 44.5±5 |
| Location of regurgitant jet | |
| A2-P2 | 9 (41) |
| A1-A2-P1-P2 | 3 (13.6) |
| A2-A3-P2-P3 | 10 (45.5) |
| Other associated valvulopathies (not mitral stenosis) | 11 (50) |
| Anteroposterior diameter, mm | 28.27±4.9 |
| Intercommissural diameter, mm | 40.68±4.7 |
EROA, effective regurgitant orifice area; PISA, proximal isovelocity surface area; TAPSE, tricuspid annular plane systolic excursion.
Values are expressed as no. (%) or mean±standard deviation.
In our series, only 1 device was required for a satisfactory result in 16 patients, whereas it was decided to implant a second clip to reduce the degree of baseline regurgitation in 6 patients. Five of these were implanted in the same procedure and 1 was implanted in a second procedure, after it was observed at follow-up that the regurgitation had progressed from grade II to grade III, with an associated significant progressive clinical deterioration. Regarding the baseline measurements of the mitral annulus evaluated using 3D transesophageal echocardiography, the anteroposterior diameter of the mitral annulus before release of the device was 28.27 mm±4.9mm and the intercommissural diameter was 40.68 mm±4.7mm.
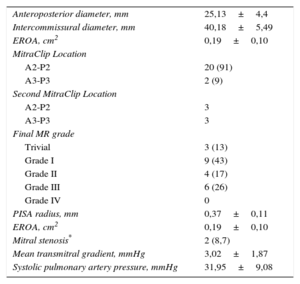
After release of the device, the anteroposterior diameter was significantly reduced by 3.14 mm±1.01mm (P<.0005), with no changes observed in the intercommissural diameter (0.50 mm±0.91 mm; P=.26). The other echocardiographic parameters analyzed after device release are shown in Table 3.
Postimplant Echocardiographic Parameters (n=22)
| Anteroposterior diameter, mm | 25,13±4,4 |
| Intercommissural diameter, mm | 40,18±5,49 |
| EROA, cm2 | 0,19±0,10 |
| MitraClip Location | |
| A2-P2 | 20 (91) |
| A3-P3 | 2 (9) |
| Second MitraClip Location | |
| A2-P2 | 3 |
| A3-P3 | 3 |
| Final MR grade | |
| Trivial | 3 (13) |
| Grade I | 9 (43) |
| Grade II | 4 (17) |
| Grade III | 6 (26) |
| Grade IV | 0 |
| PISA radius, mm | 0,37±0,11 |
| EROA, cm2 | 0,19±0,10 |
| Mitral stenosis* | 2 (8,7) |
| Mean transmitral gradient, mmHg | 3,02±1,87 |
| Systolic pulmonary artery pressure, mmHg | 31,95±9,08 |
MR, mitral regurgitation; EROA, effective regurgitant orifice area; PISA, proximal isovelocity surface area.
Values are expressed as no. (%) or mean±standard deviation.
Regarding the intraobserver and interobserver variability in the intercommissural and anteroposterior diameters, the intraclass correlation coefficients were 0.96 (95% confidence interval [95%CI], 0.92-0.99) and 0.96 (95%CI, 0.91-0.99) for the calculation of intraobserver variability, and 0.96 (95%CI, 0.91-0.98) and 0.97 (95%CI, 0.92-0.99) for the calculation of interobserver variability (Figure 2). Bland-Altman plots showed the absence of systemic bias and adequate limits of agreement for the measurements (Figure 2).
The EROA was significantly reduced immediately after device implantation (0.30 cm±0.04cm2; P<.0005) from a baseline value of 0.49cm2±0.09cm2.
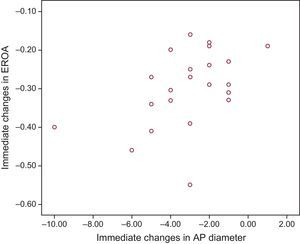
Lastly, a significant relationship was observed between the reduction in regurgitant orifice area and the reduction in the anteroposterior diameter assessed using 3D transesophageal echocardiography (r=0.49; 95%CI, 0.05-0.73; P=.020) (Figure 3).
These results coincided when analyzed taking into account the percentage reduction in diameter (r=0.51; 95%CI, 0.11-0.76; P=.014). A clear trend toward statistical significance was seen when the analysis excluded the 2 extreme values observed (1 case of a 10mm reduction in the anteroposterior diameter and 1 case of an increase) (r=0.43; 95%CI, 0.03-0.72; P=.059).
When coefficients were calculated from the linear regression model of the association between the change in EROA and the change in the anteroposterior diameter of the mitral annulus, the constant was 0.24 (-0.31 to -0.17) and the coefficient was 0.20 (0.002-0.04).
DISCUSSIONAlthough most of the patients treated with this device in the pivotal EVEREST II trial had organic etiology,9 subsequent “real world” experience has shown that, undeniably, patients treated with this device have MR of predominantly functional etiology.16–18 This device is therefore a good treatment option for patients with symptoms of heart failure despite optimal medical treatment, who often have contraindications for surgery.5,6 In our series, 23 of the 29 patients treated in our hospital (79.3%) had functional mitral regurgitation.
As previously mentioned, the mechanism of action of the percutaneous procedure is similar to that of the Alfieri technique,8 approximating and joining the mitral leaflets, generally at the central part of the valve, to reduce the degree of regurgitation. However, unlike the Alfieri technique, the percutaneous device is not combined with ring implantation or annuloplasty. The outcomes of patients undergoing the surgical Alfieri technique without annuloplasty have been demonstrated to be clearly worse that those who undergo both techniques, especially in patients with remodeled or calcified annuluses.10,19,20 This may be because the mitral leaflets are exposed to increased systolic and diastolic stress in patients without associated annuloplasty.21
Thus, this may be a disadvantage of the percutaneous technique. However, in patients with functional MR who receive percutaneous treatment, our study shows that annulus remodeling occurs immediately after release of the device, with a reduction in the anteroposterior diameter; this could have an effect similar to that of annuloplasty.
Although there are few data on this subject in the literature, other authors have also reported mitral annulus remodeling immediately after percutaneous mitral repair in functional regurgitation, due to the reduction in anteroposterior diameter; this finding has not been demonstrated in organic MR.11 This may be because the anteroposterior diameter is close to normal in organic regurgitation but is clearly increased in functional MR, as in our patients, whose mitral annulus diameter measurements were comparable to those published in other series.11
Regarding the reduction in mitral regurgitation severity, as far as we know, no study has demonstrated a correlation between annulus remodeling and the degree of reduction of regurgitation in patients with functional MR. This is probably because in the few existing studies, both etiologies are analyzed together,11 while the mechanisms of regurgitation are entirely different.
In our series, to evaluate MR as accurately as possible, all parameters from current guidelines5,6 on the evaluation of MR were integrated, with the EROA as the parameter of choice when we studied the correlation between the reduction in MR and annulus remodeling.
Undoubtedly, the evaluation of MR after percutaneous mitral repair is complex, given the presence of the double orifice. Currently, there is no consensus for this evaluation, nor are there established data on the subject in the literature. In our series, as in other series, the analysis of post-implant EROA was based on the sum of the EROAs of the 2 regurgitant orifices,15 after images acquisition in which the 2 regurgitation jets were sufficiently separated with no interference.
Quantitative MR assessment by 3D analysis of the vena contracta is an emergent potential method for MR assessment in different situations, including the assessment of regurgitation after percutaneous mitral repair. In the quantification of MR, a good correlation has been demonstrated between the vena contracta of both orifices and the evaluation of this regurgitation with cardiac magnetic resonance; thus, this may be a more accurate method.22
The results obtained in this study provide useful information on the mechanism of action of this percutaneous device in functional MR. Percutaneous techniques have advanced enormously in the treatment of MR. In the near future, annulus remodeling could be used to guide decision-making regarding which patients could benefit from percutaneous mitral ring implantation and which patients would not require it due to significant annulus remodeling following implantation of the MitraClip device.23
This study analyzed the changes in the mitral annulus produced immediately after implantation of 1 or more percutaneous devices; it did not assess the relationship between these changes and the patients’ clinical outcomes. However, other authors have demonstrated that a lesser reduction in the anteroposterior diameter and a greater degree of residual MR after the procedure are associated with worse clinical outcomes and greater need for reintervention.24,25
Therefore, it would be interesting to ascertain whether this reduction in the anteroposterior diameter is maintained in the mid- to long-term. Such a maintained effect could occur due to constant traction of the fibrotic tissue created around the device, which would contribute to maintaining the immediate results. The results of the 4-year follow-up of the patients in the EVEREST II study were consistent with this: they showed that patients not requiring reintervention in the first year maintained the good immediate results in terms of the grade of mitral regurgitation26 and clinical stability in line with the degree of MR reduction.
LimitationsThis was a single-center study with a small number of patients, and there was no control group. Furthermore, new 3D echocardiography software now exists, allowing more complete assessment of the mitral annulus with measurements of not only the diameters, but also the circumference and area of the mitral annulus, which could provide more complete information on mitral annulus remodeling.
CONCLUSIONSPercutaneous mitral valve repair leads to an immediate reduction in the anteroposterior diameter of the mitral annulus in patients with functional MR, without changes in the intercommissural diameter. This remodeling may be related to the immediate reduction in mitral regurgitation evaluated according to the EROA.
CONFLICTS OF INTERESTM. Pan and J. Suárez de Lezo have received payment from Abbott for the development of lectures, including participation in the speakers’ bureau. A. López has received payment from Abbott for the development of educational presentations and expert testimony.
- -
There are few published studies analyzing the mechanism of action and changes produced in the mitral annulus after percutaneous implantation of the MitraClip device. The data obtained in this study on mitral annulus remodeling due to the reduction in the anteroposterior diameter in patients with functional mitral regurgitation are comparable to those reported by other authors. However, as far as we know, no study has analyzed the correlation between this remodeling and the reduction in mitral regurgitation.
- -
Although this study has a low statistical power, as mentioned above, as far as we know it is the first study to demonstrate a probable relationship between annulus remodeling due to the reduction in the anteroposterior diameter and a reduction in mitral regurgitation. This relationship, observed immediately, could provide important information and even help in the near future in making decisions during the procedure, such as the final position of the clip or even the possibility of combining it with a percutaneous mitral ring.