Keywords
INTRODUCTION
Numerous studies have highlighted the increased risk for cardiovascular complications in patients who have experienced heart failure (HF) during the index hospitalization for high-risk non-ST segment acute coronary syndromes (NSTEACS).1-4 The implementation of more aggressive therapies in this subgroup of patients, including high utilization of revascularization procedures, has been associated with a consistent reduction in adverse outcomes, including death and heart failure.5,6 Nevertheless, little is known about the incidence, clinical risk factors and prognostic implications of an episode of rehospitalization for acute heart failure (AHF) in patients who have survived to the index hospitalization for high-risk NSTEACS. In this regard, very few studies have been conducted to explore the burden associated with a post-discharge AHF episode after an acute myocardial infarction.7-9 These authors have found that HF, when it occurred after a myocardial infarction (MI), was associated with an increased risk of mortality7-9; however, the association between HF and the risk for subsequent MI was not sought.
Moreover, the extrapolation of these findings to patients with high-risk NSTEACS is limited. Most of these patients have had an index episode of ST-segment elevation myocardial infarction (STEMI),7-9 and therefore carry higher risk for subsequent development of HF. In addition, most of these studies were done in the context of clinical trials,10 with results that may apply only to a selected population with more stable disease. For instance, patients in the early post-discharge period—when the risk of cardiovascular events is higher—were excluded.7,8 Furthermore, no information was provided on how the risk of developing a new acute ischemic event, particularly MI, is modified when a post-discharge AHF preceded such event.
We thus believe that characterizing the occurrence of intermediate endpoints such as AHF and how it modifies the natural history of NSTEACS will be of paramount importance, with potential therapeutic implications. Therefore, we designed this study in order to determine the risk of subsequent mortality and MI associated with the development of AHF following a hospitalization for high-risk NSTEACS.
METHODS
Study Population and Protocol
We studied prospectively a cohort of 1017 patients consecutively admitted to our institution from January 2001 to May 2005 with a diagnosis of high-risk NSTEACS. NSTEACS was defined by the presence of typical chest pain within the preceding 24 hours with elevation in Troponin I (TnI) and/or ST-segment depression on the electrocardiogram. Our final cohort for analysis included 972 patients, after excluding 45 in-hospital deaths. Detailed medical history, vital signs, 12-lead electrocardiogram, and routine lab measurements were obtained on admission. TnI was measured at admission, and serially every 8-12 hours, based on patient condition. Every patient was treated with aspirin and low molecular weight heparin subcutaneously for 5-7 days unless percutaneous coronary intervention (PCI) was planned. Therapy with beta-blockers, was strongly recommended, whereas concomitant therapy with nitrates, angiotensin converting enzyme inhibitors, statins, calcium channel blockers, and angiotensin-II receptor blockers was left at the discretion of the attending physician. Clopidogrel was prescribed in accordance with established guidelines during different calendar periods.11,12 Following international guidelines,12 a more invasive revascularization strategy was recommended in our institution beginning in November 2002, although the choice of the procedure remained, in the end, the responsibility of the attending physician. Patients who received an intracoronary stent were treated with clopidogrel and aspirin as recommended in the established guidelines.11,12 Usage of abciximab was reserved for approved indications and administered only in the catheterization laboratory 10 to 60 minutes before the first balloon inflation. Left ventricular ejection fraction (EF) was assessed with 2-dimensional transthoracic echocardiography during index hospitalization.
All endpoints for this study were ascertained during post-discharge follow-up after the index hospitalization for high-risk NSTEACS. AHF and MI were defined following corresponding guidelines.13,14 In all cases patients were hospitalized and treatment with intravenous diuretics, inotropes, or vasodilators was initiated for clinical stabilization. MI complicated by HF during the first 72 hours was counted as MI and not as AHF. The diagnosis was initially established by trained cardiologists and confirmed during hospitalization. Patients' clinical status was routinely evaluated either during recurrent hospitalizations or ambulatory clinic visits. This study was approved by an institutional review committee and patients gave informed consent.
Statistical Analysis
T-test and χ2 test were used for the comparison between baseline variables and occurrence of post-discharge AHF. The cumulative incidence function (CIF) plots for MI and mortality (final endpoints) were estimated according to AHF status (intermediate endpoint), and their differences tested by the Gray test.15
For assessing the association between AHF and MI with subsequent death, as well as the association between AHF with subsequent MI, a time-varying Cox proportional hazards models was used.16 For the analysis where MI was the last endpoint, patients were followed-up until the first episode, ignoring repeated episodes that occurred afterwards. Candidate covariates (all variables listed in Table 1) for multivariable analysis were chosen based on previous medical knowledge, and independent of their P-value. Then, a parsimonious, highly predictive model was derived by using backward stepdown selection,17 with at least a 10:1 ratio between the numbers of covariates per events. The functional form of continuous variables in the log-hazard scale was examined by means of fractional polynomials18 and transformed accordingly. A 2-sided P-value of <.05 was considered statistically significant for all analyses, which were performed using STATA 10.1 and the cmprsk package in R.
RESULTS
Baseline Characteristics of the Population
The mean age of our sample was 68 (12) years with 64.3% males. HF during index admission (Killip >I), TnI elevation and ST-segment depression were present in 15.6%, 84.9%, and 47.2% of patients, respectively.
Eighty-two (8.4%) episodes of AHF were ascertained at follow-up. Patients with AHF were older (mean age, 77 vs 68 years) and more frequently female (51% vs 34%). Likewise, AHF occurred more frequently in association with diabetes, previous ischemic heart disease, chronic renal failure, peripheral artery disease, history of aspirin use, and treatment with aldosterone antagonist (Table 1). Conversely, a lower prevalence of current smoking, family history of coronary heart disease (CHD), PCI performed during the index hospitalization, clopidogrel prescription at discharge, and/or lower glomerular filtration rate (GFR) were documented in these patients (Table 1). Likewise, mean value of EF was lower in the subgroup that further developed AHF.
Incidence Pattern of Post-Discharge Endpoints
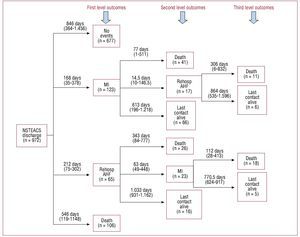
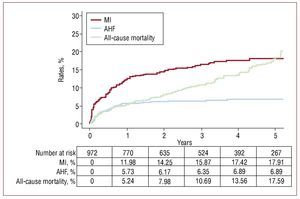
The total median follow-up was 30 months (interquartile range, 12-48). Eighty-two patients (8.4%) had AHF, 146 (15%) had an MI, 47 (4.8%) underwent PCIs; 38 (3.9%) underwent CABGs at follow-up, and 202 (20.8%) patients died. Sequence and level of events are shown in Figure 1. Rehospitalization for AHF and MI occurred more frequently during the first year, whereas all-cause mortality showed a monotonic increase throughout the follow-up (Figure 2). The median (interquartile range) time elapsed from NSTEACS discharge to the first admission for AHF was 203 days (interquartile range, 56-336) and only 17 (18.3%) cases of AHF episodes occurred following an episode of post-discharge acute MI (Figure 1).
Figure 1. Sequence and level of events (MI, AHF, and death) occurred following a high-risk NSTEACS. AHF indicates acute heart failure; MI, myocardial infarction; NSTEACS, non-ST segment acute coronary syndrome.
Figure 2.Incidence rates for the first cardiovascular outcomes (whichever occurred first) following discharge for a high-risk NSTEACS. AHF indicates acute heart failure; MI, myocardial infarction; NSTEACS, non-ST segment acute coronary syndrome.
Predictors of Rehospitalization for Acute Heart Failure
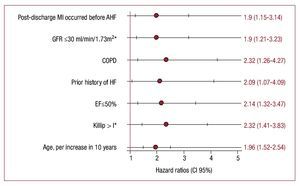
Independent of traditional risk factors (Figure 3), postdischarge MI becomes an independent predictor of AHF (HR, 1.9; 95% CI, 1.15-3.14; P=.013).
Figure 3.Predictors of post-discharge admission for AHF following a high-risk NSTEACS. Model stratified by gender and year of admission. COPD indicates chronic pulmonary obstructive disease; EF, left ventricular ejection fraction; GFR, estimated glomerular filtration rate (Modification of Diet in Renal Disease formula); HF, heart failure; MI, myocardial infarction; NSTEACS, non-ST segment acute coronary syndrome.
Rehospitalization for Acute Heart Failure as a Predictor of Mortality
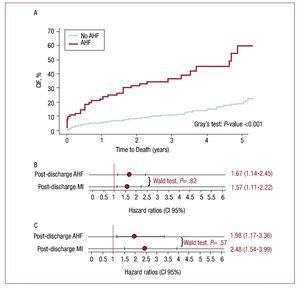
After an admission for AHF, 55 (67.1%) patients died. Patients with AHF experienced higher mortality rates (Figure 4A). In a univariate analysis, patients with AHF showed a 5-fold increased risk of mortality. This association diminished, although significance persisted, after adjusting for baseline risk factors and MI as a time-varying covariate (HR, 1.67; 95% CI, 1.14-2.65; P=.009) (Figure 4B). The baseline covariates included in the final model were age, gender, calendar year, dyslipidemia, diabetes mellitus, family history of coronary artery disease, previous stroke, peripheral artery disease, previous use of aspirin, Killip >I, GFR, EF<50%, revascularization procedures performed at the index hospitalization (PCI or coronary artery bypass graft during index hospitalization) and treatment with statins at discharge. In a sensitivity analysis, excluding patients with HF during index admission (Killip >I), AHF remained independently associated with subsequent mortality (Figure 4C).
Figure 4. Post-discharge time varying adverse outcomes (AHF and MI) and subsequent death. A: CIF of mortality by post-discharge hospitalization for AHF. B: post-discharge events (AHF and MI) and risk of subsequent death in whole sample. C: post-discharge events (AHF and MI) and risk of subsequent death excluding patients with Killip >I during index admission. Model stratified by gender and year of admission and adjusted for the following covariates: age, gender, calendar year, dyslipidemia, diabetes mellitus, family history of coronary artery disease, previous stroke, peripheral artery disease, previous use of aspirin, Killip >I, glomerular filtration rate, left ventricular ejection fraction <50%, PCI and CABG surgery during index hospitalization, and treatment with statins at discharge. AHF indicates acute heart failure; CABG, coronary artery bypass graft; CIF, cumulative incidence function; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Rehospitalization for Acute Heart Failure as a Predictor of Myocardial Infarction
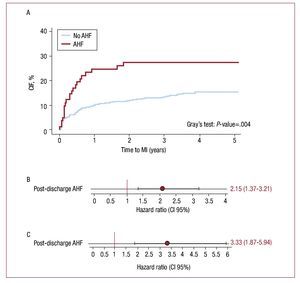
Following the episode of AHF, 23 (28%) patients experienced a subsequent MI. The CIF curves showed that AHF was associated with increased risk for MI with notorious differences observed from the first year (Figure 5A). In univariate analysis, AHF was associated with a 4.2-fold increase in risk for MI. After adjusting for age, gender, calendar year, hypertension, diabetes mellitus, smoking status, peripheral artery disease, troponin I elevation, Killip >I, GFR, EF<50%, PCI, and CABG during index hospitalization, AHF remained independently associated with MI (HR, 2.15; 95% CI, 1.41-3.27; P<.001) (Figure 5B). In a sensitivity analysis where patients with HF at the index admission (Killip >I) were excluded from the analysis, AHF remained an important predictor of MI in univariate as well as in multivariate analysis (HR, 3.33; 95% CI, 1.87-5.94; P<.001) (Figure 5C).
Figure 5. Post-discharge acute heart failure and subsequent myocardial infarction. A: CIF of MI by post-discharge hospitalization for AHF. B: post-discharge AHF and risk of subsequent MI. C: post-discharge AHF and risk of subsequent MI excluding patients with Killip >I during index admission. Model stratified by gender and year of admission and adjusted for the following covariates: age, hypertension, diabetes mellitus, smoking status, peripheral artery disease, troponin I elevation, Killip >I, glomerular filtration rate, left ventricular ejection fraction <50%, PCI and CABG during index hospitalization. AHF indicates acute heart failure; CABG, coronary artery bypass graft; CIF, cumulative incidence function; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Prognostic Implications of Post-Discharge Myocardial Infarction
As expected, MI was associated with increased risk of mortality under univariate (HR, 4.09; 95% CI, 3.03-5.49; P<.001) as well as multivariate analysis (HR, 1.56; 95% CI, 1.11-2.22; P=.011). When the 2 HR for mortality were compared (AHF vs MI), no differences were found (P=.821) (Figure 3B).
DISCUSSION
In the present study, we showed that the first post-discharge episode of AHF modifies the natural history of high-risk NSTEACS by significantly increasing the risk of subsequent MI or death. This association remained significant after adjusting for well-known prognostic risk factors including HF and left ventricular EF during index admission. Furthermore, whereas ours and previous results19 have highlighted that MI is a risk factor for subsequent AHF, based on our results we propose that development of AHF constitutes a risk factor for incident MI. Our findings underscore the need to reassess the diagnostic and therapeutic approach to these patients.
Prognostic Limitations of Baseline Stratification Models
Statistical prediction models for CV outcomes in patients with ACS have been traditionally performed as a one-time assessment of risk factors. The tradeoff associated with a time-varying modelling is the ability to capture important variations in baseline risk that may occur when intermediate endpoints (such as new MI or ACS) arise.20,21
Acute Heart Failure as a Critical Warning Sign
HF is a well-established complication in patients with ischemic heart diseases, especially immediately following an episode of NSTEACS.1-4 Despite the fact that ischemic heart disease is recognized as a risk factor for HF development, rehospitalization for AHF has not been commonly assessed as a component of the primary endpoint in this population. Recently, there has been a renewed interest in identifying baseline clinical risk factors associated with late development of AHF, and underscoring its importance as a prognostic factor for subsequent death in post-MI patients.7-9 A sub-study of the CARE8 trial included 85% of patients with Q-wave MI, with a mean time inclusion after MI of 10 months; patients were excluded if they had symptomatic HF. The VALIANT7 trial included 70% of patients with Q-wave MI; patients were excluded if they had previous HF, normal EF and cardiovascular outcomes within the first 45 days. These 2 studies reported a significant increase in mortality, in a time-dependent fashion, when an episode of AHF occurred after an MI post-discharge. Furthermore, and in agreement with our results, both studies reported similar baseline (age, left ventricular EF, glomerular filtration rate) and time-varying (recurrent MI) predictors of AHF.
Our study, to the best of our knowledge, is the first in assessing the risk burden associated with a new admission for AHF in non-selected, high-risk NSTACS patients including the early post-discharge follow-up, during which time the risk for cardiovascular complications is higher.22,23 We found that: a) the incidence of AHF after a high-risk NSTEACS is high, mainly during the first year (Figure 2); b) AHF is not always a direct consequence of a simultaneous MI; c) AHF predicted the development of MI; and d) the risk of death associated with AHF was similar in magnitude to the risk of death after having an MI.
The independent association found between AHF and death is an expected finding, since recent registries have reported a 1-year mortality rate around 30%-35%.24 More unexpected, however, was the independent association with subsequent MI. Orn et al,25 recently found that recurrent MI, confirmed by necropsy, was the most frequent cause of death (57%) in patients with MI and left ventricular systolic dysfunction. Similar results have been confirmed in other registries4 where Killip >I during index hospitalization was independently associated with long-term MI. Several mechanisms may be postulated to explain this association. For instances, patients with HF usually display a greater extension of coronary heart disease along with a large amount of stunned and hibernated myocardium26; in this study, we found that post-discharge AHF was most prevalent among patients with multivessel compared to monovessel disease (7.5% vs 2.7%; P=.005), among patients in which coronariography was performed (n=606). We also believe that high proinflammatory activity that usually accompanies HF may also plays a role.27,28 Thus, the findings of this study challenge a widely accepted view of HF as being the final stage in the continuum of the ischemic heart disease, where a large amount of necrotic myocardium predominates. If patients with repeated hospitalization for AHF are at high risk for subsequent MI, some degree of viable myocardium (in stunned or hibernating state) may still exist. Moreover, in a recent publication from the CHARM29 study that include 7599 patients with chronic HF class II-IV and followed-up for 3.3 years, the authors found that new admission for MI occurred in 5.82% with a subsequent increased risk of mortality within the first 30 days.
Even though clinical guidelines have strongly recommended a thorough evaluation for myocardial ischemia, invasive revascularization —when appropriate— and intense secondary prevention in patients with ACS complicated by HF, these are not routinely performed in patients with AHF and coronary artery disease.30 The evaluation process of AHF in patients with ischemic heart disease, especially following an NSTEACS, is not well defined.29,30 Current guidelines state that there are no data from multicenter trials supporting the routine evaluation for myocardial ischemia or reevaluation with subsequent revascularization in these types of patients.30 This is reflected in our sample by the fact that only 7 (8.54%) patients who were admitted for AHF underwent coronariography, and 6 of them were revascularized (3 PCIs and 3 CABG). As our results have shown, an episode of AHF after a high-risk NSTEACS has a similar prognostic burden for death and MI compared to a new episode of ACS. We, therefore, propose: a) AHF should be incorporated as a usual outcome in patients with ACS and; b) further controlled studies should assess the merits of a comprehensive evaluation for myocardial ischemia, including ischemic stress and myocardial viability tests, to optimize the anti-ischemic treatment and consider the possibility of revascularization. Along this line, recent articles have suggested that revascularization may have prognostic benefit in terms of mortality reduction in patients with AHF and coronary artery disease31 and in presence of viable myocardium.32
Strengths and Limitations
This is the first study describing the prognostic implication of post-discharge hospitalization for AHF in a large and representative population of patients with high-risk NSTEACS. We minimized the risk of confounding by including time-varying covariates in addition to baseline risk factors such as Killip >I during the index admission, left ventricular EF, revascularization procedures, and pharmacological treatment at discharge.
In addition to the inherent limitations of being an observational study, some limitations need to be addressed: a) patients with milder or subclinical forms of HF were not identified, in principle because they did not seek hospitalization; therefore, we could not rule out a step-function relationship between degrees of HF severity and incidence of MI and mortality; b) the absence of prognostic biomarkers in the prognostic models (BNP) is due to the fact that they were not routinely collected; c) some variables such as EF were only collected at baseline but not over time; d) for repeated intermediate endpoints, we followed patients until the first event of its class, due to limitation of the registry; e) in order to minimize the potential bias induced by the gap in calendar year—also called "cohort effect"— all regression models were stratified by "year of admission"; we don't know, however, the degree of residual confounding and how it may biasing our estimates. This is particularly true in relation to revascularization procedures and the use of pharmacologic treatment; f) we also recognized that, by not accounting for mortality as a competing event in the MI Cox model, some degree of bias may be operating on the estimated association between AHF rehospitalization and incidence of MI; and g) despite having adjusted for main drug classes at baseline, and because information about dosages, patient compliance or changes in treatment at follow-up were not collected, we cannot definitively rule out some degree of inaccuracy or bias in our estimates.
CONCLUSIONS
AHF is a frequent complication following a high-risk NSTEACS. Rehospitalization for AHF is a clinical event that was found to be independently associated with an increased risk of subsequent death and MI.
ABBREVIATIONS
AHF: acute heart failure
CHD: coronary heart disease
EF: ejection fraction
GFR: glomerular filtration rate
HF: heart failure
MI: myocardial infarction
NSTEACS: non-ST segment acute coronary syndromes
PCI: percutaneous coronary intervention
This study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, RED HERACLES RD06/0009/1001 (Madrid, Spain).
Correspondence: Dr. J. Núñez.
Servicio de Cardiología. Hospital Clínico Universitario. Avda. Blasco Ibáñez, 17. 46010 Valencia. Spain
E-mail: yulnunez@gmail.com
Received October 5, 2009.
Accepted for publication February 2, 2010.