Since ancient times, innovation and medicine have always gone hand in hand. The introduction and management of an innovation should be understood within the social context and the health care system in which it is developed or applied. Knowledge and analysis along these lines will allow the design of strategies and generate the initiatives that will guarantee that those innovations that truly represent a valuable contribution reach the health care system without becoming a threat to its sustainability. An analysis of the context identifies 3 major factors that can act as accelerators or decelerators of innovation. The first is the growing sophistication of medical innovations, which often entails meeting considerable human, structural, organizational, and economic requirements. The second is the difficult balance between access to the market of innovations and the demonstration of their value in routine practice. The third is the deceleration in the percentage of the gross domestic product devoted to health care spending observed in recent years in many developed countries. To continue to ensure and preserve scientific and medical progress in Spain, it is necessary to rethink and redesign our model for the provision and funding of innovation in health care.
Despite the creation of Red Española de Agencias de Evaluación de Tecnologías Sanitarias y Prestaciones del Sistema Nacional de Salud1 as a support to aid the Interregional Council in decision-making on the incorporation of medical innovations into the service portfolio of the publicly-funded Spanish health system, there is still wide variability from one region to another in terms of the characteristics of the introduction and use of many innovations. One reason for this is that the demand for the incorporation of health technologies arises much sooner in health care centers than in technology assessment agencies. By way of example, a study carried out in Denmark demonstrated that only a third of the technologies requested over the course of a year by professionals in Danish hospitals had been evaluated by the Danish agency for health technology assessment.2 The characteristics of the organization and funding of the Spanish health system allow any technological innovation labeled with the Conformité Européenne marking to be marketed for sale in Spain. Health care centers often have to decide whether or not to incorporate an innovation despite their lack of access to the most reliable or complete information on which to base the decision. Scientific societies are a key instrument for the identification of health care innovations, as their members keep abreast of scientific and technological advances and can thus assume a proactive role with respect to a given innovation. In addition, if the aim of the evaluation of an innovation of any type is to achieve results that will be adopted by those who are going to use the new technology, it requires the involvement of professional specialists in the entire process.3 For all the above reasons, it seems obvious that the role of scientific societies is crucial in ensuring the proper introduction of innovations into the Spanish health system. Therefore, the Spanish Society of Cardiology (Sociedad Española de Cardiología [SEC]) should consider itself a key factor and a reference in the introduction of technological innovations in the area of heart disease and, thus, has created InnovaSEC.
InnovaSEC is a strategic initiative of the SEC that is designed to provide criteria and tools to encourage and facilitate the orderly introduction of highly valuable innovative solutions (systems, health products, and drugs) in the area of heart disease into the Spanish health system, likewise defining the role of the SEC in this process. For this purpose, the governing body of the SEC constituted the InnovaSEC Scientific Committee, which was entrusted with drafting the criteria and principles that should oversee the decision of the committee concerning the suitability of introducing a specific innovation into Spanish health care centers. The members of the committee represent different areas of technological experience in cardiology (M. Larman, A. San Román, F. Worner Diz, J. Brugada), as well as knowledge and experience in the assessment of health technology innovations (L. Sampietro-Colom). The analysis of each of the criteria selected by the InnovaSEC Scientific Committee should be based on internationally recognized methodological guidelines for health technology assessment and take into consideration the specific characteristics of each particular innovation. Given that the concept of innovation in cardiology—such as, which parameters determine that the new technology is a valuable contribution to the health system—can have different meanings, the first task of the InnovaSEC Scientific Committee was to define the concepts “innovation” and “value-adding innovation” in the field of cardiology.
DEFINITION OF INNOVATION IN THE InnovaSEC CONTEXTThe definition of innovation can range from a generic definition to one adapted to the productive sector that it targets. Generic definitions of innovation include the sense of the novelty of an idea, method, or product4,5 that is going to be made commercially available for the first time and for which there are paying customers.6 It also includes the concept of offering substantial advantages over existing products and/or covers needs that currently have no available solutions.7
The definition of innovation transferred to the health care setting contextualizes the concepts of generic definitions. Thus, to be an innovative solution in the health care context, it is necessary to satisfy the following conditions: a) the innovative products should address unmet needs and improve health outcomes; b) the innovations should be novel, should offer improvements over existing products, and should constitute a step forward in terms of patient outcomes; and c) an innovation should provide treatment for a condition with no existing treatment or effective intervention or, if a possible intervention is available, it does not achieve the desired levels of efficacy.8 From all these definitions, a lowest common denominator can be extracted: novelty, improvement in health outcomes, and response to an unmet need.8
Based on the various definitions, the InnovaSEC Scientific Committee defines health care innovation as the application of novel solutions that respond to new objectively identified requirements or to real needs that are not covered and/or that offer improvements in health outcomes, from the point of view of both patients and professionals and the health system in general, or improve the existing options for addressing a health problem.
“VALUE”-ADDING INNOVATION ACCORDING TO InnovaSECDetermination of whether or not a technological innovation adds value to current practice can vary from one country to another and within any given country, depending on who assigns this value. Every country has its own culture and social values, which determine the importance allotted to each of the attributes of the innovation itself and to the results it produces. Thus, the same innovation can be considered to be a valuable contribution or otherwise in different countries. In turn, depending on the profile of the person or entity defining the value of the innovation (patient, health authority, clinician, industry), the criteria to be considered and the relative importance assigned to each in the final decision will also contribute to determining whether or not a technological innovation is considered to add value.9
The InnovaSEC Scientific Committee has defined the value of an innovation from the point of view of health care professionals, but has also taken into account the perspective of the patients, which the professionals are aware of because of their day-to-day relationship, and that of the health authorities, to whom professionals are increasingly obliged to account for their administration of the resources they use. Thus, InnovaSEC considers that a technological innovation adds value if it improves health status, enhances quality of life, and/or reduces the mortality rate with respect to the best available options, at an acceptable cost.
IDENTIFICATION OF InnovaSEC CRITERIA FOR THE ASSESSMENT OF INNOVATIONSThe identification of the criteria that are to govern the proper introduction of innovations of value in the field of cardiology into the Spanish health system was carried out in several stages and using different methodologies. First, the tables of contents of the key journal in health technology assessment (the International Journal of Technology Assessment in Health Care) were reviewed. One study was found to mention 42 criteria of interest employed to assess the introduction of a technological innovation. The study was the result of an international survey involving 140 professionals (one third clinicians, two thirds professional health policy makers), from 23 countries on 5 continents, who customarily decide on the introduction of health innovations in their context.10 For the purpose of identifying any other relevant criteria not identified in that article, a literature search was performed in the PubMed and EMBASE databases, yielding 979 articles, whose abstracts were reviewed by 2 members of the InnovaSEC Scientific Committee. Of all these articles, only 3 studies mentioned a new criterion that had not been included in the aforementioned article (unit price of the drug). Likewise, we reviewed the document drawn up by European Network for Health Technology Assessment (EUnetHTA), which provides the criteria that should be considered in the evaluation of new technologies in the medical and surgical fields,11 but it did not identify any additional new criteria.
From these information sources, we obtained 2 lists. The first consisted of 55 criteria related to technological innovation and the second consisted of 5 criteria related to the characteristics of the centers that were interested in adopting the innovation. Each member of the InnovaSEC Scientific Committee reviewed the lists individually in order to: a) evaluate the suitability of each criterion for its application in the case of innovations in the area of heart disease, and b) identify new criteria (1 new criterion identified: transparency in the reporting and auditing of results). Subsequently, the committee discussed the assessments face to face and a consensus was reached on the final list of criteria. After this consensus, the initial 60 criteria were reduced to 21 (15 directly related to the innovation and 6 to the centers wishing to introduce it). Tables 1 and 2 show the resulting InnovaSEC criteria that are to be taken into account for the introduction of innovations in the field of heart disease in the Spanish health system.
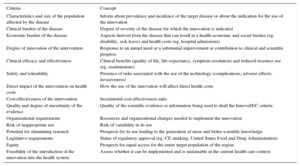
InnovaSEC Criteria Related to Health Innovation
| Criteria | Concept |
|---|---|
| Characteristics and size of the population affected by the disease | Inform about prevalence and incidence of the target disease or about the indication for the use of the innovation |
| Clinical burden of the disease | Degree of severity of the disease for which the innovation is indicated |
| Economic burden of the disease | Aspects derived from the disease that can result in a health-economic and social burden (eg, disability, sick leave) and health costs (eg, hospital admissions) |
| Degree of innovation of the intervention | Response to an unmet need or a substantial improvement or contribution to clinical and scientific progress |
| Clinical efficacy and effectiveness | Clinical benefits (quality of life, life expectancy, symptom resolution) and reduced resource use (eg, readmissions) |
| Safety and tolerability | Presence of risks associated with the use of the technology (complications, adverse effects, invasiveness) |
| Direct impact of the intervention on health costs | How the use of the innovation will affect direct health costs |
| Cost-effectiveness of the intervention | Incremental cost-effectiveness ratio |
| Quality and degree of uncertainty of the evidence | Quality of the scientific evidence or information being used to draft the InnovaSEC criteria |
| Organizational requirements | Resources and organizational changes needed to implement the innovation |
| Risk of inappropriate use | Risk of variability in its use |
| Potential for stimulating research | Prospects for its use leading to the generation of more and better scientific knowledge |
| Legislative requirements | Status of regulatory approval (eg, CE marking, United States Food and Drug Administration) |
| Equity | Prospects for equal access for the entire target population of the region |
| Feasibility of the introduction of the innovation into the health system | Assess whether it can be implemented and is sustainable in the current health care context |
CE, Conformité Européenne.
InnovaSEC Criteria Related to the Centers in Which the Innovation Is to Be Introduced
| Criteria to be taken into account | Concept |
|---|---|
| Training requirements | Characteristics and level of training necessary to enable the use of the innovation |
| Learning curve | Experience in the area in which the innovation is to be implemented, number of cases involving similar procedures, need for instruction from experts in the innovation |
| Reliability and capability of the team | Previous experience in similar procedures and requirements (necessary services and technologies) related to the innovation |
| Workflow and quality indicators | Minimum necessary workflow |
| Transparency in outcome reporting | Commitment of the center to report information on the process and the results of applying the innovation |
| Information for the patient and informed consent | Clear selection criteria and informed consent |
The assessment process for the incorporation of innovation will be governed by a series of principles that guarantee the credibility and reliability of the evaluation carried out, of the decision-making process, and of the final decision. The assessment of any innovation should be systematic, structured, evidence-based, unbiased, and robust. The assessment should be based on the quantitative and qualitative methods exhibiting the best agreement with the characteristics of the technology being evaluated and with the assessment question to be addressed. These methods should be the product of the best scientific knowledge available at the time of the evaluation. Likewise, the assessment should take into account the characteristics of the Spanish health care context. The decision-making process will be based on transparency, which implies reporting the sources on which the assessment and its entire process is based, as well as stating explicitly how and why the final decision has been reached, allowing for the presentation of arguments for the decision prior to its implementation.
The InnovaSEC Scientific Committee will be the SEC's reference point for any issue related to innovation. Its main objective is to assess the proposals for the introduction of innovations into the health system by the SEC, considering the information associated with the criteria defined in this editorial and respecting the principles mentioned in the preceding paragraph. The functions of the committee include: a) the identification and proactive assessment of innovations (prospective technology); b) responding to the demand for the assessment of technological innovations within the SEC; c) participation in planning the incorporation of these innovations; and d) establishing the characteristics of the process of reporting the results of the centers incorporating the innovation, if necessary. The committee is multidisciplinary and its members must declare any conflicts of interest they may have each time the committee is called on to act.
The recommendations of the InnovaSEC Scientific Committee will be classified as: a) positive: the innovation meets the conditions that make it an innovation of value for patients and for the Spanish health system; b) positive with monitoring: the state of development of the innovation and the information evaluated indicate that it is highly probable that it meets the conditions that make it an innovation of value for patients and the Spanish health system, but the results of the assessment are not conclusive and, thus, monitoring of the introduction is advisable; c) introduction with evidence development: the information on the innovation is limited and unconvincing, but there are positive indications (the innovation should be subjected to an investigation protocol that will provide the information required by the SEC and the health authorities); and d) negative: the characteristics of the state of development or of the innovation itself or of the health care context do not favor any of the above recommendations at the present time.
CONCLUSIONSThe SEC has no intention of interfering with the fundamental role of the Red Española de Agencias de Evaluación de Tecnologías Sanitarias y Prestaciones del Sistema Nacional de Salud, but understands that, in the cardiology setting, the scientific societies in general and the SEC in particular can offer an additional, and highly useful, professional view when it comes to making the final decision on the introduction of technological innovations. Both the criteria and the process defined for InnovaSEC lay the foundations for the participation of the SEC in good governance of the introduction of value-adding innovations in the field of cardiology into the Spanish health system.
CONFLICTS OF INTERESTNone declared.
We thank our fellow members of the InnovaSEC Scientific Committee: Mariano Larman, Alberto San Román, and Fernando Worner.