Although pulmonary valve stenosis (PVS) is considered a low risk congenital heart disease, there have been reports of complications and the need for reintervention throughout follow-up. The aims of this study were to evaluate the long-term outcome of repaired PVS and to identify predictors of cardiovascular complications and reintervention.
MethodsWe studied 158 adult patients with repaired PVS (repair procedures performed from 1957 to 2010) receiving active follow-up in a tertiary referral center.
ResultsA total of 95 patients (60%) received surgical treatment, and 63 patients (40%) received percutaneous pulmonary balloon valvuloplasty. At the end of follow-up (27 years, IQR, 20-33 years), most patients (n=134, 84.8%) were in New York Heart Association functional class I, but 61 patients (38.6%) required a reintervention, mainly pulmonary valve replacement (17.7%, n=28), and 19 patients (12%) had at least one cardiovascular complication: 13 (8.2%) supraventricular arrhythmias, 6 (3.8%) heart failure, 5 (3.2%) stroke, 1 (0.6%) death, 1 (0.6%) thromboembolism, and 1 (0.6%) ventricular arrhythmia. Multivariate analysis showed that age at PVS repair (HR, 1.08; 95%CI, 1.04-1.12; P <.001) and the presence of cyanosis before PVS repair (HR, 5.23; 95%CI, 1.99-13.78; P=.001) were independent predictors for cardiovascular complications.
ConclusionsGood long-term outcome can be expected after PVS repair, but complications and the need for reintervention may appear. Older age and the presence of cyanosis at PVS repair emerged as predictors of cardiovascular complications and identified a population that may merit stricter control.
Keywords
Pulmonary valve stenosis (PVS) is a common cause of right ventricular outflow tract obstruction that affects 7% to 12% of patients with congenital heart disease.1 When technically feasible, percutaneous balloon valvuloplasty is currently the strategy of choice but there is a huge population of patients in follow-up that underwent surgical repair in earlier eras. Overall, PVS is considered a low risk congenital heart disease with near normal life expectancy,2–4 but a number of cardiac events (predominantly supraventricular arrhythmias) may appear throughout follow-up.5 There is, however, a paucity of published data addressing this issue. In addition, pulmonary regurgitation is a common finding in the long-term and a need for reintervention may ensue. The aim of this study was to evaluate the long-term outcome of repaired PVS and to identify predictors of cardiovascular complications and reintervention.
METHODSTwo hundred eleven patients (≥ 16 years) with repaired PVS were identified using a prospectively maintained database of the congenital heart disease unit of a single tertiary referral center. Of these, 158 patients were receiving active follow-up at the same center or at peripheral congenital cardiac units (at least 1 visit in the preceding 5 years) and constituted the study population. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the human research committee of our institution. PVS repair procedures were performed between 1957 and 2010, predominantly at our institution, and included surgical treatment in 95 patients (60%, from 1957) and percutaneous pulmonary balloon valvuloplasty in 63 patients (40%, from 1985). Patients with associated congenital heart defects other than atrial septal defect (ASD), patent foramen ovale (PFO) or patent ductus arteriosus were excluded. Demographic data, preoperative information, surgical details and last follow-up data (including clinical status, electrocardiogram, echocardiography, and cardiac magnetic resonance when available) were retrospectively obtained from the patients records. Right atrial enlargement on the ECG was considered when the P wave height measured> 2.5mm (2.5mV) in inferior leads and> 1.5mm in V1 or, in the absence of ECG tracings, when recorded as such in the clinical records. Cyanosis was collected as a dichotomous variable and considered positive when O2 saturation was <90% or, in the absence of O2 saturation data, when clinical records stated so based on physical examination. Events of interest were reintervention (considered as any cardiac surgery or percutaneous treatment of a residual defect performed after the initial repair) and cardiovascular complications defined as a composite endpoint of cardiovascular death, heart failure (clinical signs of right heart congestion), arrhythmia (sustained supraventricular tachycardia and sustained/nonsustained ventricular tachycardia), stroke, and embolism. Pulmonary artery (PA) dilation was considered when the PA trunk measured ≥ 40mm by an imaging technique (echocardiography, computed tomography, or cardiac magnetic resonance). Hemodynamic data were completed using catheterization records. Echocardiographic data were revised from patients’ records. Studies were performed and reported according to the current European Echocardiography Guidelines at the moment of the diagnostic test. Pulmonary regurgitation and tricuspid regurgitation were objectively evaluated according to the parameters described in the guidelines.
Continuous variables are described as mean±standard deviation and as median and interquartile range (IQR) when a normal distribution could not be demonstrated. Categorical variables are described as percentages. Comparisons between variables were performed by the Student t test, chi-square test or nonparametrical Mann-Whitney U-test and Fisher's exact test as appropriate. Cox proportional hazard models were performed to quantify the bivariate association between baseline variables and outcomes of interest (cardiovascular complications and need for reintervention). All the variables with statistical significance in the bivariate analysis <0.2 were selected for multivariable modelling, which included forward and backward stepwise methods with a threshold for exit set at P higher than .10 and for entering at P lower than .10. Proportional hazard assumption was graphically checked. Interaction between variables was tested.
Survival free of cardiovascular complications and need for reintervention was estimated using Kaplan-Meyer curves for the surgical and percutaneous valvuloplasty cohorts and was compared by log-rank test. Patients were censored when the first event occurred.
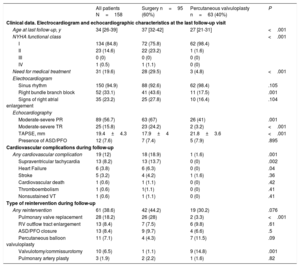
RESULTSBaseline data: general overviewSurgical treatment was more frequent in our cohort as a whole, predominantly in the early years. The most frequently performed surgical procedure was valvulotomy (n=92; 92.8%) with or without infundibulectomy in 41 (43.1%) patients. Three patients (3.1%) underwent valvulectomy. Additional ASD/PFO closure was performed in 20 patients (21%), transannular patch in 6 (6.3%) and ventriculotomy was required in 12 (12.6%).
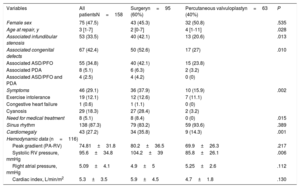
Table 1 contains all the demographic and preoperative data of the entire cohort and in 2 subgroups according to the type of procedure performed (surgery or valvuloplasty). The median age at repair was 3 years (IQR, 1-7 years) in the group as a whole. About one third of the cohort (n=55, 34.8%) had associated ASD/PFO and approximately a third of the patients (n=46, 31.1%) had symptoms before PVS repair (either cyanosis, congestive heart failure, arrhythmia, or exercise intolerance).
Baseline (preoperative) data
| Variables | All patientsN=158 | Surgeryn=95 (60%) | Percutaneous valvuloplastyn=63 (40%) | P |
|---|---|---|---|---|
| Female sex | 75 (47.5) | 43 (45.3) | 32 (50.8) | .535 |
| Age at repair, y | 3 [1-7] | 2 [0-7] | 4 [1-11] | .028 |
| Associated infundibular stenosis | 53 (33.5) | 40 (42.1) | 13 (20.6) | .013 |
| Associated congenital defects | 67 (42.4) | 50 (52.6) | 17 (27) | .010 |
| Associated ASD/PFO | 55 (34.8) | 40 (42.1) | 15 (23.8) | |
| Associated PDA | 8 (5.1) | 6 (6.3) | 2 (3.2) | |
| Associated ASD/PFO and PDA | 4 (2.5) | 4 (4.2) | 0 (0) | |
| Symptoms | 46 (29.1) | 36 (37.9) | 10 (15.9) | .002 |
| Exercise intolerance | 19 (12.1) | 12 (12.6) | 7 (11.1) | |
| Congestive heart failure | 1 (0.6) | 1 (1.1) | 0 (0) | |
| Cyanosis | 29 (18.3) | 27 (28.4) | 2 (3.2) | |
| Need for medical treatment | 8 (5.1) | 8 (8.4) | 0 (0) | .015 |
| Sinus rhythm | 138 (87.3) | 79 (83.2) | 59 (93.6) | .389 |
| Cardiomegaly | 43 (27.2) | 34 (35.8) | 9 (14.3) | .001 |
| Hemodynamic data (n=116) | ||||
| Peak gradient (PA-RV) | 74.81±31.8 | 80.2±36.5 | 69.9±26.3 | .217 |
| Systolic RV pressure, mmHg | 95.6±34.8 | 104.2±39 | 85.8±26.1 | .006 |
| Right atrial pressure, mmHg | 5.09±4.1 | 4.9±5 | 5.25±2.6 | .112 |
| Cardiac index, L/min/m2 | 5.3±3.5 | 5.9±4.5 | 4.7±1.8 | .130 |
ASD, atrial septal defect; PA-RV, pulmonary artery-right ventricle; PDA, patent ductus arteriosus; PFO, patent foramen ovale; RV, right ventricular.
Data are presented as mean±standard deviation, No. (%), or median [interquartile range].
Statistically significant differences were observed between patients undergoing surgery compared with those undergoing percutaneous valvuloplasty, in terms of age at time of PVS repair (4.8±7.0 vs 8.3±12.4; P=.028), systolic pressure of right ventricle (RV) (104.2±39 vs 85.8±26mmHg; P=.006), presence of symptoms (41% vs 16.7%; P=.002) and, therefore, need for medical treatment (9.3% vs 0.0%; P=.015). However, the severity of stenosis (peak RV - PA gradient): 80.2±36.5 vs 69.9±26.3mmHg; P=.217) was not significantly different. As expected, patients undergoing surgical repair more frequently had associated infundibular stenosis (42.1% vs 20.6%; P=.013) or ASD/PFO or patent ductus arteriosus requiring closure (52.6% vs 27%; P=.010).
Last follow-up data: general overviewAfter a median follow-up of 27 years (QR, 20-33 years, surgical cohort 32 years [IQR, 28-36 years], percutaneous valvuloplasty 20 years [IQR, 17-23 years]; P <.001), most patients were in New York Heart Association functional class I and only 31 patients (19.6%) needed medical treatment (either beta-blockers, digoxin, diuretics, oral anticoagulation, or antiarrhythmic drugs). Most of them were in sinus rhythm (n=150; 94.9%) and right bundle branch block was present in 52 (33.1%) with a global mean QRS duration of 107.7±23.5ms (135±15.6ms for those with right bundle branch block). Echocardiography evaluation in the last follow-up visit showed residual pulmonary stenosis with peak gradient ≥ 36mmHg (39±3mmHg) in only 3% (n=5) of patients. Moderate to severe pulmonary regurgitation was present in more than a half of the cohort (n=89; 56.7%) at the end of follow-up and moderate to severe right ventricular dilation was seen in 39 (24.7%). For patients requiring reintervention with pulmonary valve replacement, the peak gradient through the prosthetic valve was 22.8±8.3mmHg with only mild-or-mild to moderate pulmonary regurgitation.
Forty-six pregnancies were registered among 31 women in our cohort, and a single patient had complications before delivery. A 30-year-old patient with history of PVS surgically repaired at 3 days of life, with subsequent development of severe pulmonary regurgitation, presented at 36 weeks of gestation with cyanosis and peripheral oedema. Complementary tests showed restrictive RV pattern and the presence of an ostium secundum ASD near the entrance of the inferior vena cava. The patient was prescribed bed rest and some fluid restriction and delivered at 38 weeks with no further complications.
Heart disease in the offspring was detected in only 1 case (mild PVS).
Last follow-up data: comparison between surgical and percutaneous cohortsStatistically significant differences were found between the surgical group and the percutaneous valvuloplasty group in terms of the presence of right bundle branch block (43.6% vs 17.5%; P=.001) and, therefore, QRS duration (115±25ms vs 96±15ms; P <.001). Systolic function of the RV evaluated by tricuspid annular plane systolic excursion (17.9±4mm vs 21.8±3.6mm; P <.001) was statistically significantly different between both groups. The remaining data related to the last follow-up visit is summarized in Table 2.
Follow-up data
| All patients N=158 | Surgery n=95 (60%) | Percutaneous valvuloplasty n=63 (40%) | P | |
|---|---|---|---|---|
| Clinical data. Electrocardiogram and echocardiographic characteristics at the last follow-up visit | ||||
| Age at last follow-up, y | 34 [26-39] | 37 [32-42] | 27 [21-31] | <.001 |
| NYHA functional class | <.001 | |||
| I | 134 (84.8) | 72 (75.8) | 62 (98.4) | |
| II | 23 (14.6) | 22 (23.2) | 1 (1.6) | |
| III | 0 (0) | 0 (0) | 0 (0) | |
| IV | 1 (0.5) | 1 (1.1) | 0 (0) | |
| Need for medical treatment | 31 (19.6) | 28 (29.5) | 3 (4.8) | <.001 |
| Electrocardiogram | ||||
| Sinus rhythm | 150 (94.9) | 88 (92.6) | 62 (98.4) | .105 |
| Right bundle branch block | 52 (33.1) | 41 (43.6) | 11 (17.5) | .001 |
| Signs of right atrial enlargement | 35 (23.2) | 25 (27.8) | 10 (16.4) | .104 |
| Echocardiography | ||||
| Moderate-severe PR | 89 (56.7) | 63 (67) | 26 (41) | .001 |
| Moderate-severe TR | 25 (15.8) | 23 (24.2) | 2 (3.2) | <.001 |
| TAPSE, mm | 19.4±4.3 | 17.9±4 | 21.8±3.6 | <.001 |
| Presence of ASD/PFO | 12 (7.6) | 7 (7.4) | 5 (7.9) | .895 |
| Cardiovascular complications during follow-up | ||||
| Any cardiovascular complication | 19 (12) | 18 (18.9) | 1 (1.6) | .001 |
| Supraventricular tachycardia | 13 (8.2) | 13 (13.7) | 0 (0) | .002 |
| Heart Failure | 6 (3.8) | 6 (6.3) | 0 (0) | .04 |
| Stroke | 5 (3.2) | 4 (4.2) | 1 (1.6) | .36 |
| Cardiovascular death | 1 (0.6) | 1 (1.1) | 0 (0) | .42 |
| Thromboembolism | 1 (0.6) | 1(1.1) | 0 (0) | .41 |
| Nonsustained VT | 1 (0.6) | 1 (1.1) | 0 (0) | .41 |
| Type of reintervention during follow-up | ||||
| Any reintervention | 61 (38.6) | 42 (44.2) | 19 (30.2) | .076 |
| Pulmonary valve replacement | 28 (18.2) | 26 (28) | 2 (3.3) | <.001 |
| RV outflow tract enlargement | 13 (8.4) | 7 (7.5) | 6 (9.8) | .61 |
| ASD/PFO closure | 13 (8.4) | 9 (9.7) | 4 (6.6) | .5 |
| Percutaneous balloon valvuloplasty | 11 (7.1) | 4 (4.3) | 7 (11.5) | .09 |
| Valvulotomy/commissurotomy | 10 (6.5) | 1 (1.1) | 9 (14.8) | .001 |
| Pulmonary artery plasty | 3 (1.9) | 2 (2.2) | 1 (1.6) | .82 |
ASD, atrial septal defect; NYHA, New York Heart Association; PFO, patent foramen ovale; PR, pulmonary regurgitation; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; VT, ventricular tachycardia.
Data are presented as median [interquartile range] or No. (%).
Median age at last follow-up was 34 years (IQR, 26-39 years). PA dilation ≥ 40mm was present in 14 patients (8.8%) with a mean diameter of 45.2±6.8mm (maximum diameter 60mm). A patient with a PA of 60mm and severe pulmonary regurgitation developed angina due to left main coronary compression. After PVR and PA resection (interposition of a valved conduit), the compression and its symptoms resolved.
No episodes of endocarditis were documented.
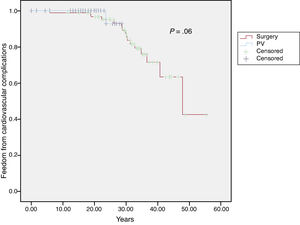
At the end of follow-up, 19 patients (12%) had a cardiovascular complication (as previously defined). Survival free of cardiovascular complications at 10 and 20 years was 99% and 96%, respectively, in the surgical cohort and 100% and 93%, respectively, in the percutaneous valvuloplasty cohort (P=.6) (Figure 1).
The individual components of the composite endpoint are outlined in Table 2. There was only 1 death in our series. A 78-old-year man had PVS repair consisting of valvulotomy and infundibular resection at the age of 35 years. At 65 years, permanent atrial fibrillation was diagnosed and 1 year later the patient showed progressively worsening New York Heart Association functional class and signs of peripheral congestion. A moderate-to-severe pulmonary regurgitation was documented with moderate RV dilation and mild depressed systolic RV function. The subsequent clinical course was fatal due to refractory heart failure and death.
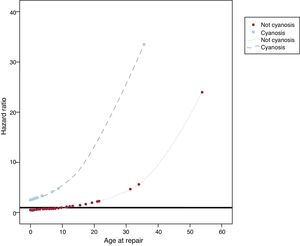
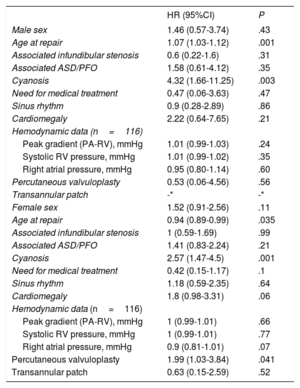
The only variables associated with cardiovascular complication in the bivariate analyses (Table 3) were age (HR, 1.07; 95%CI, 1.03-1.12; P <.001) and cyanosis at PVS repair (HR, 4.32; 95%CI, 1.66-11.25; P=.003). Both variables remained predictors for cardiovascular complications on multivariable analysis (age at PVS repair: HR, 1.08; 95%CI, 1.04-1.12; P <.001, cyanosis: HR, 5.23; 95%CI, 1.99-13.78; P=.001). Moreover, a statistically significant interaction between the 2 variables emerged (P <.001), which can be seen in Figure 2. The relationship between age at intervention and cardiovascular events depended on the presence of cyanosis. In patients with cyanosis, there was a higher risk of cardiovascular complications regardless of age at repair (although there was a higher risk in those repaired at an older age). In contrast, in patients without cyanosis, age at repair was associated with a lower risk of cardiovascular events in those undergoing surgery at a young age, whereas the risk progressively increased in those patients undergoing surgery at older ages.
Bivariate associations between baseline characteristics and long-term cardiovascular complications and need for reintervention
| HR (95%CI) | P | |
|---|---|---|
| Male sex | 1.46 (0.57-3.74) | .43 |
| Age at repair | 1.07 (1.03-1.12) | .001 |
| Associated infundibular stenosis | 0.6 (0.22-1.6) | .31 |
| Associated ASD/PFO | 1.58 (0.61-4.12) | .35 |
| Cyanosis | 4.32 (1.66-11.25) | .003 |
| Need for medical treatment | 0.47 (0.06-3.63) | .47 |
| Sinus rhythm | 0.9 (0.28-2.89) | .86 |
| Cardiomegaly | 2.22 (0.64-7.65) | .21 |
| Hemodynamic data (n=116) | ||
| Peak gradient (PA-RV), mmHg | 1.01 (0.99-1.03) | .24 |
| Systolic RV pressure, mmHg | 1.01 (0.99-1.02) | .35 |
| Right atrial pressure, mmHg | 0.95 (0.80-1.14) | .60 |
| Percutaneous valvuloplasty | 0.53 (0.06-4.56) | .56 |
| Transannular patch | -* | -* |
| Female sex | 1.52 (0.91-2.56) | .11 |
| Age at repair | 0.94 (0.89-0.99) | .035 |
| Associated infundibular stenosis | 1 (0.59-1.69) | .99 |
| Associated ASD/PFO | 1.41 (0.83-2.24) | .21 |
| Cyanosis | 2.57 (1.47-4.5) | .001 |
| Need for medical treatment | 0.42 (0.15-1.17) | .1 |
| Sinus rhythm | 1.18 (0.59-2.35) | .64 |
| Cardiomegaly | 1.8 (0.98-3.31) | .06 |
| Hemodynamic data (n=116) | ||
| Peak gradient (PA-RV), mmHg | 1 (0.99-1.01) | .66 |
| Systolic RV pressure, mmHg | 1 (0.99-1.01) | .77 |
| Right atrial pressure, mmHg | 0.9 (0.81-1.01) | .07 |
| Percutaneous valvuloplasty | 1.99 (1.03-3.84) | .041 |
| Transannular patch | 0.63 (0.15-2.59) | .52 |
95%CI, 95% confidence interval; ASD, atrial septal defect; HR, hazard ratio; PA-RV, pulmonary artery-right ventricle; PFO, patent foramen ovale; RV, right ventricular.
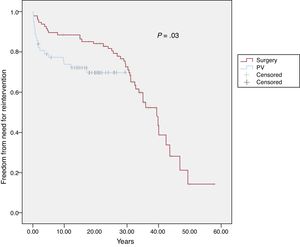
After PVS repair, a total of 61 patients (38.6%) required additional procedures, 10 of them (16.4% of the reintervention group) required 2 reinterventions and 4 of them (6.5% of the reintervention group) ≥ 3 reinterventions. In addition, 5 of these patients required either pacemaker/defibrillator implantation or ablation procedures at the time of the reintervention or during follow-up. Survival free of need for reintervention at 10, 15 and 20 years was 88%, 87%, and 85%, respectively, in the surgical cohort and 74%, 72% and 70%, respectively, in the percutaneous valvuloplasty cohort (P=.03) (Figure 3).
The most frequent procedures were pulmonary valve replacement, ASD/PFO closure, and right outflow tract enlargement techniques. Information related to reintervention is described in Table 2. In the bivariate analyses (Table 3) need for reintervention was associated with younger age at PVS repair (HR, 0.94; 95%CI, 0.89-0.99; P=.035), the presence of cyanosis prior to repair (HR, 2.57; 95%CI,1.47-4.5; P=.001), and the use of percutaneous valvuloplasty (HR, 1.99; 95%CI, 1.03-3.84; P <.041). In the multivariate analysis, the presence of cyanosis prior to repair (HR, 3.17; 95%CI,1.68-5.97; P <.001) and the use of percutaneous valvuloplasty (HR, 3.48; 95%CI,1.67-7.25; P=.001) remained predictors for reintervention. The most frequent reintervention in patients undergoing initial percutaneous valvuloplasty for PVS repair was surgical comisurotomy/valvotomy (Table 2). As seen in the Kaplan-Meyer curves, the need for reintervention in the percutaneous valvuloplasty group occured soon after the initial procedure and in the early era of the procedure (57% of the 14 patients who underwent percutaneous valvuloplasties between 1985-1988 required reintervention, whereas only 21% of the remaining 48 patients did so, P=.02).
DISCUSSIONThis series comprises a tertiary referral center cohort of patients with isolated PVS repaired either with surgery or percutaneous valvuloplasty. After a median follow-up of 27 years, although the average survival rate and functional class were good, 38.6% of patients required reintervention and 12% had cardiovascular complications.
Several studies have reported excellent outcomes in patients with repaired PVS,2–4,6 with good functional class at the end of follow-up, as in our study, and survival similar or only slightly shorter than those in the general population.4,7 Nonetheless, complications and, mainly, the need for reintervention have been described during follow-up.2–6
The reported need for reintervention varies widely among the various series.3,8 Kopecky et al.,4 in their surgical series published in 1988 (191 patients, 24.4 years of follow-up), observed a need for reintervention of only 2.6%.In 2005, the same group reported a reintervention rate of 53%, mostly related to pulmonary regurgitation, in a selected cohort of 53 patients drawn from the adult congenital cardiac unit database (follow-up 33 years).3 Other contemporary series reporting results after percutaneous balloon valvuloplasty also show a variable rate of reintervention (2%-30%), mainly related to residual PVS.9,10
In a cohort of 158 patients (60% surgical and 40% percutaneous) and after a median follow-up of 27 years, we report a reintervention rate of 38.6%. This variability in the reported reintervention rate may be explained by several factors: the difference in length of follow-up between series, the different techniques used for PVS relief, and the evolving criteria for reintervention of the residual lesions, particularly pulmonary regurgitation. Moreover, some series include as reinterventions the implantation of pacemakers/defibrillators or ablation techniques, whereas others do not. As for predictors of reintervention, the literature points to technical issues at the time of initial PVS repair: the use of transannular patch3 and closed valvotomy5 in surgical patients and the balloon to annulus ratio in percutaneous patients.10 In our series, despite the surgical cohort accounting for a larger number of reinterventions, as shown in Table 1, percutaneous valvuloplasty emerged as a risk factor for reintervention after adjustment for time of follow-up. In view of the association of the need for reintervention with the early era of the procedure, however, this may be interpreted as a “failed strategy” of percutaneous valvuloplasty during the learning curve of the technique itself and candidate selection. More intriguing is the relationship of cyanosis prior to PVS repair with the need for reintervention.
While several articles in the literature elaborate on the need for reintervention, there is a paucity of data on predictors of cardiac complications after PVS relief. In a comprehensive report published in 1988, Kopecky et al.4 focused on the long-term complications of their surgical series (median follow-up of 24.3 years) and reported cardiovascular events in 20 patients (10.4%): cardiovascular death in 11, congestive heart failure in 2, reinterventions for residual pulmonary stenosis in 5, and pacemaker implantations in 2. Independent predictors for such events were advanced age at time of repair and higher preoperative and postoperative right ventricular pressure. Although there is some information about long-term complications in the recent literature, there is no other study focusing on predictive factors. In our contemporary cohort of 158 patients with active follow-up (median 27 years) in a tertiary referral congenital cardiac unit, 19 patients (12%) had cardiac complications, namely cardiovascular death, heart failure, arrhythmia, stroke, and embolism. The multivariate analysis identified cyanosis and older age at repair as the factors likely associated with late cardiovascular complications.
The relationship between older age at repair and cardiovascular complications may reflect the deleterious effect of long-term right ventricular pressure overload and the subsequent ventricular hypertrophy and myocardial fibrosis. Right ventricular fibrosis is well recognized to occur as a result of severe pulmonary stenosis and it increases progressively with age to be universally present by the age of 30 or older.11 Recent studies examining the presence of restrictive RV physiology by cardiac magnetic resonance have found a strong association with fibrosis evaluated by late gadolinium enhancement.12 In adults with tetralogy of Fallot, extensive presence of late gadolinium enhancement in the RV was related to adverse clinical markers, including decreased exercise tolerance and clinical arrhythmia.13–15 Currently, arrhythmia is the major driver of our composite endpoint of cardiovascular complications.
Ross-Hesselink et al.3 reported recurrent supraventricular tachycardia in only 3 patients (3.3%). In an update of that cohort The same group,10, described only an additional patient with atrial arrhythmias. The authors do not describe complex ventricular arrhythmias in their surgical cohort but report nonsustained ventricular tachycardia in 2% of Holter monitoring and 1 case of sudden cardiac death 25 years after surgical repair. Sudden cardiac death has been seldom reported in other series.6,8
The other predictor of cardiovascular complications in our study was the presence of cyanosis before PVS relief, as proposed by the group of Helen Taussig back in 1963.16 Moreover, in our study, a statistically significant interaction was detected between both predictors so that the relationship between age at repair and the risk of cardiovascular events was somehow mediated by the presence of cyanosis, which conferred a poor prognosis at any age.
Cyanosis is attributed to a right-to-left shunt through a PFO. Although it may reflect more severe pulmonary stenosis, when assessing the parameters of severity of the lesion (the RV systolic pressure and the pulmonary valve gradient), no association was found with the occurrence of cardiovascular complications. It can be speculated that cyanosis reflects a worse myocardial adaptation to the maintained pressure overload situation in the neonatal period and during the early stages of life. The intrinsic mechanisms of this unfavorable myocardial adaptation (possibly also related to RV diastolic dysfunction) may persist after PVS relief, leading to a higher rate of cardiac complications and need for reintervention.
LimitationsThe main limitation of this study is its retrospective nature. Of the 211 patients with repaired PVS identified in our database, only 158 were receiving active follow-up and constituted the study population. The search for information was mainly done through the medical records of our center or referral cardiologists and, in some cases, based on primary physician electronic records. Although a 25% loss to follow-up should be considered by all means excessive, recent studies report rates of up to 42% of gaps in care among patients with adult congenital heart disease, 8% of which lasted for more than 10 years17. The main reason for discontinuation of care in these studies, regardless the cardiac complexity, was the fact that patients felt well and did not feel that further follow-up was necessary.
The length of follow-up of the surgical and the percutaneous valvuloplasty group were significantly different. Although percutaneous valvuloplasty emerged as a predictor of need for reintervention, this finding was based on the statistical assumption that the risk for reintervention of both populations remains constant through time and, as shown in the Kaplan-Meir curves, this might not be the case.
CONCLUSIONSPatients with repaired PVS had an excellent postoperative outcome. Nonetheless, complications and the need for reintervention may appear in the long-term. PVS repair at an older age and the presence of cyanosis before PVS relief, both factors probably reflecting a worse right ventricular myocardium profile after repair, emerged as predictors of unfavorable outcome and identified a population that may merit closer follow-up.
FUNDINGThis study was funded by CIBERCV and co-financed by the European Regional Development Fund (ERDF-FEDER).
CONFLICTS OF INTERESTNone declared.
- -
Repaired PVS is considered a low-risk congenital cardiac condition with near-normal life expectancy. However, there have been reports of long-term complications after PVS repair.
- -
Long-term outcome was reviewed in a contemporary population of repaired PVS patients. Reintervention was required in 38% of the patients and cardiovascular complications occurred in 12%. The main finding of our study was that older age at repair and, more importantly, cyanosis prior to repair were independent predictors of the composite endpoint of cardiovascular complications (cardiovascular death, heart failure, arrhythmia, stroke, and embolism).