The vasomotor function of new-generation drug-eluting stents designed to enhance stent healing and reendothelialization is unknown. This study aimed to compare the endothelial function of the infarct-related artery (IRA) treated with bioactive circulating endothelial progenitor cell-capturing sirolimus-eluting stents (COMBO) vs polymer-free biolimus-eluting stents (BioFreedom) in ST-segment elevation myocardial infarction patients at 6 months. Secondary objectives were to compare the microcirculatory function of the IRA and stent healing at 6 months.
MethodsSixty patients were randomized to bioactive sirolimus-eluting stent vs polymer-free biolimus-eluting stents implantation. At 6 months, patients underwent coronary angiography with vasomotor, microcirculatory and optical coherence tomography examinations. Endothelial dysfunction of the distal coronary segment was defined as ≥ 4% vasoconstriction to intracoronary acetylcholine infusion.
ResultsEndothelial dysfunction was similarly observed between groups (64.0% vs 62.5%, respectively; P=.913). Mean lumen diameter decreased by 16.0 ±20.2% vs 16.1 ±21.6% during acetylcholine infusion (P=.983). Microcirculatory function was similar in the 2 groups: coronary flow reserve was 3.23 ±1.77 vs 3.23±1.62 (P=.992) and the index of microcirculatory resistance was 24.8±16.8 vs 21.3±12.0 (P=.440). Optical coherence tomography findings were similar: uncovered struts (2.3% vs 3.2%; P=.466), malapposed struts (0.1% vs 0.3%; P=.519) and major evaginations (7.1% vs 5.6%; P=.708) were observed in few cases.
ConclusionsEndothelial dysfunction of the IRA was frequent and was similarly observed with new-generation drug-eluting stents designed to enhance stent reendothelialization at 6 months. Endothelial dysfunction was observed despite almost preserved microcirculatory function and complete stent coverage. Larger and clinically powered studies are needed to assess the role of residual endothelial dysfunction in ST-segment elevation myocardial infarction patients.
Registered in ClinicalTrials.gov: NCT04202172
Keywords
Primary percutaneous coronary intervention is the preferred reperfusion strategy in patients with ST-segment elevation myocardial infarction (STEMI). Endothelial function of the infarct-related artery (IRA) is often impaired in the contiguous distal segment.1 Endothelial dysfunction after drug-eluting stent (DES) implantation has been associated with persistent angina and adverse clinical outcomes.2
New-generation DES aim to enhance stent healing and re-endothelialization. Bioactive sirolimus-eluting stents (SES) (COMBO, OrbusNeich, The Netherlands) combine an abluminal bioabsorbable sirolimus-coated polymer with an adluminal CD34+antibody layer designed to capture circulating endothelial progenitor cells. In a preclinical swine model, bioactive SES showed a larger degree of strut re-reendothelialization than durable polymer DES at 14 days.3 The polymer-free biolimus A9-eluting stent (BES) (BioFreedom, Biosensors, Switzerland) is designed to release the antiproliferative drug a few days after stent implantation.4 For this reason, BES are considered to have similar re-endothelialization to bare metal stents (BMS). However, the epicardial and microcirculatory vasomotor function of the IRA treated with new-generation DES designed to enhance stent reendothelialization is still unknown.
The primary objective of the present study was to describe and compare the endothelial function of the distal IRA segment treated with bioactive SES (COMBO) vs polymer-free BES (BioFreedom) at 6 months. Secondary objectives were to describe and to compare the microcirculatory function and stent healing of the 2 devices at 6 months.
METHODSStudy design and populationThis study is an investigator-initiated, descriptive, proof of concept, pivotal, multicenter, randomized trial promoted by the Spanish Society of Cardiology and funded by Orbus Neich (The Netherlands). The funding source and the promoter of the study had no role in the study design, data management, data analysis, or final report.
The inclusion and exclusion criteria of the study are detailed in the methods of the supplementary data. In summary, all informed STEMI patients with suitable clinical and anatomical conditions for enrollment in the study were randomized 1:1 to bioactive SES (COMBO) vs polymer-free BES (BioFreedom). Patients were randomized if they had Thrombolysis in Myocardial Infarction ≥ 2 flow after wire crossing, predilatation, or thrombus aspiration according to the operator's criteria. Antiplatelet and antithrombotic therapy was left to the operator's criteria according to the standard procedures of each participating Institution. All patients included in the study were requested to undergo a new coronary angiogram, as per protocol, at 6 months. The study was performed according to the provisions of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of each participating center. Written informed consent was obtained from all patients.
Six-month invasive coronary procedurePatients were requested to stop all vasomotor drugs at least 24hours before coronary angiography. Vasomotor drugs were not allowed before the vasomotor test in case the radial approach was used.
The 6-month invasive protocol consisted of 3 parts. First, an epicardial vasomotor test of the IRA was performed to assess the endothelial-dependent and endothelial-independent responses of the distal coronary segment. Endothelial-dependent function was examined by intracoronary infusion of acetylcholine at incremental doses of 10-6 M and 10-4 M according to previous publications.5 Acetylcholine infusion was given via a microcatheter (Teleport, OrbusNeich, The Netherlands) at least 5mm proximal to the proximal stent edge. The endothelial-independent function was investigated by bolus injection of 200μg of intracoronary nitroglycerin via a guiding catheter. A detailed explanation of the vasomotor test can be found in the methods of the supplementary data.
Second, microcirculatory function assessment was performed with a dedicated intracoronary wire with pressure and temperature sensors (PressureWire X Guidewire, Abbott, United States). According to previous publications,5 the index of microcirculatory resistance, coronary flow reserve and fractional flow reserve were performed under intravenous adenosine infusion (140μg/kg/min).
Finally, optical coherence tomography (OCT) imaging was performed with a dedicated catheter (Dragonfly OPTIS, Abbott, United States) according to standard procedures.
Angiographic analysisAngiographic analysis was performed by a core laboratory (BARCICORE-Lab, Barcelona, Spain) using specific software for quantitative coronary angiography analysis (CASS 5.9; Pie Medical BV, The Netherlands). The analysts were blinded to the study groups.
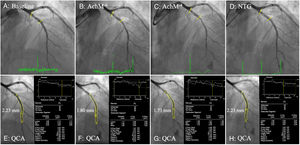
The vasomotor responses of the distal coronary segment to endothelial-dependent and independent stimuli were assessed taking into account the core laboratory variability for mean lumen diameter repeated measurements. The 2 standard deviation difference between quantitative angiographic measurements of matched coronary segments is 3.9%.5,6 Therefore, a vasoconstrictive response to low-dose or high-dose acetylcholine infusion (meaning endothelial dysfunction) was defined when ≥ 4% vasoconstriction was observed with respect to the baseline 6-month mean lumen diameter. The distal coronary segment was defined as the segment between the stent edge and up to 20 to 40mm according to natural landmarks. Assessment of vasomotor changes is shown in figure 1.
Quantitative coronary angiography analysis of the distal segment. A, B, C, D: 6-month vasomotor test angiographic images at baseline, acetylcholine doses, and nitroglycerin. Stent edges are marked with yellow lines. E, F, G, H: quantitative coronary angiogram of matched distal segments between the different vasomotor drugs. The mean lumen diameter of matched segments is shown in each respective image. NTG, nitroglycerin; QCA, quantitative coronary angiography.
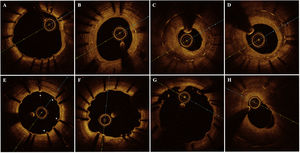
OCT analysis was performed by a core-laboratory (BARCICORE-Lab, Barcelona, Spain) using specific software for analysis (LightLab Imaging, United States). Two blinded analysts were requested to assess the following qualitative OCT findings in the entire pullback (0.2mm intervals) according to a previous study7: the neointima pattern at the cross-section with largest neointima area, observation of cross-sections with a ratio of uncovered to total stent struts ≥ 30%, presence of major coronary evaginations and neoatherosclerotic plaques. Figure 2 shows the main OCT qualitative findings observed in the study. Quantitative OCT data were analyzed each 1mm according to standard core laboratory procedures.7 A detailed description of the quantitative OCT analysis is provided in the methods of the supplementary data.
Main optical coherence tomography qualitative findings. A: absent neointima; B: homogeneous neointima; C: heterogeneous neointima; D: layered neointima; E: RUTTS (ratio of uncovered to total stent struts) ≥ 30%, uncovered struts are shown with *; F: major coronary evagination; G: incomplete stent apposition; H: fibro-lipidic neoatherosclerotic plaque.
This is a hypothesis-generating pivotal study. Therefore, there was no sample size calculation since there were no previous data regarding the endothelial function of new-generation DES. Categorical variables are presented as counts and percentages, and continuous variables as mean±standard deviation. Comparisons of categorical variables were estimated with the chi-square or Fisher exact tests, as appropriate. Comparisons of continuous variables between groups were evaluated with the Student t-test or nonparametric Mann-Whitney or Kruskal-Wallis tests, as appropriate. Comparisons of the same parameter at different time points (such as mean lumen diameter changes during the vasomotor test) were assessed with generalized linear modelling for repeated measures. OCT strut level analysis was performed considering the clustering nature of the OCT data with generalized estimation equations. All struts were classified into the following types: apposed and covered, apposed and uncovered, malapposed and covered and malapposed and uncovered. Each strut type was introduced into the model as a dependent variable using the binary logistic model. Each model was performed introducing stent type as covariate and patient identification as a subject variable. A 2-sided P value<.05 was considered statistically significant. Statistical analysis was performed with the SPSS software, version 20.0 (SPSS Inc, United States).
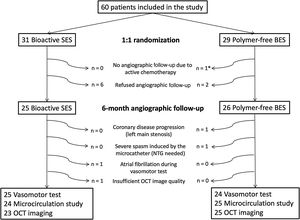
RESULTSPopulationA total of 60 patients were included (31 bioactive SES COMBO and 29 polymer-free BES BioFreedom) in 3 institutions from November 2018 to September 2019. No clinical events or unscheduled angiographic follow-ups were documented at 6 months. Eight patients refused angiographic follow-up and 1 patient was excluded due to current chemotherapy treatment. Therefore, 51 patients (25 bioactive SES and 26 polymer-free BES) underwent invasive examination, as per protocol, at 6 months.
One patient had coronary disease progression (left main stenosis) and was excluded for further invasive examinations. Another patient had symptomatic paroxysmal atrial fibrillation during acetylcholine infusion and did not undergo a microcirculatory function test and OCT imaging. The flow chart of the study is shown in figure 3.
Flow chart of the study. * 1 patient was diagnosed with colon cancer 1 month after the baseline procedure and was treated with chemotherapy during the angiographic follow-up period. This patient was excluded from invasive angiographic follow-up. BES, biolimus-eluting stent; NTG, nitroglycerin; OCT, optical coherence tomography; SES, sirolimus-eluting stent.
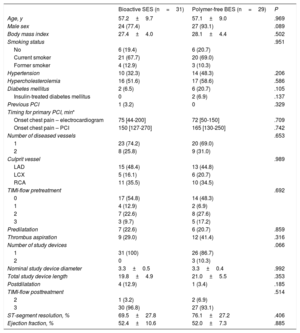
Baseline clinical and procedural characteristics are shown in table 1. The main clinical characteristics were similar between groups. Most patients had complete occlusion of the culprit vessel (54.8% bioactive SES vs 48.3% polymer-free BES; P=.692). The most common IRA was the left anterior descending artery (48.4% vs 44.8%; P=.989).
Baseline clinical and procedural characteristics
| Bioactive SES (n=31) | Polymer-free BES (n=29) | P | |
|---|---|---|---|
| Age, y | 57.2±9.7 | 57.1±9.0 | .969 |
| Male sex | 24 (77.4) | 27 (93.1) | .089 |
| Body mass index | 27.4±4.0 | 28.1±4.4 | .502 |
| Smoking status | .951 | ||
| No | 6 (19.4) | 6 (20.7) | |
| Current smoker | 21 (67.7) | 20 (69.0) | |
| Former smoker | 4 (12.9) | 3 (10.3) | |
| Hypertension | 10 (32.3) | 14 (48.3) | .206 |
| Hypercholesterolemia | 16 (51.6) | 17 (58.6) | .586 |
| Diabetes mellitus | 2 (6.5) | 6 (20.7) | .105 |
| Insulin-treated diabetes mellitus | 0 | 2 (6.9) | .137 |
| Previous PCI | 1 (3.2) | 0 | .329 |
| Timing for primary PCI, min* | |||
| Onset chest pain – electrocardiogram | 75 [44-200] | 72 [50-150] | .709 |
| Onset chest pain – PCI | 150 [127-270] | 165 [130-250] | .742 |
| Number of diseased vessels | .653 | ||
| 1 | 23 (74.2) | 20 (69.0) | |
| 2 | 8 (25.8) | 9 (31.0) | |
| Culprit vessel | .989 | ||
| LAD | 15 (48.4) | 13 (44.8) | |
| LCX | 5 (16.1) | 6 (20.7) | |
| RCA | 11 (35.5) | 10 (34.5) | |
| TIMI-flow pretreatment | .692 | ||
| 0 | 17 (54.8) | 14 (48.3) | |
| 1 | 4 (12.9) | 2 (6.9) | |
| 2 | 7 (22.6) | 8 (27.6) | |
| 3 | 3 (9.7) | 5 (17.2) | |
| Predilatation | 7 (22.6) | 6 (20.7) | .859 |
| Thrombus aspiration | 9 (29.0) | 12 (41.4) | .316 |
| Number of study devices | .066 | ||
| 1 | 31 (100) | 26 (86.7) | |
| 2 | 0 | 3 (10.3) | |
| Nominal study device diameter | 3.3±0.5 | 3.3±0.4 | .992 |
| Total study device length | 19.8±4.9 | 21.0±5.5 | .353 |
| Postdilatation | 4 (12.9) | 1 (3.4) | .185 |
| TIMI-flow posttreatment | .514 | ||
| 2 | 1 (3.2) | 2 (6.9) | |
| 3 | 30 (96.8) | 27 (93.1) | |
| ST-segment resolution, % | 69.5±27.8 | 76.1±27.2 | .406 |
| Ejection fraction, % | 52.4±10.6 | 52.0±7.3 | .885 |
BES, biolimus-eluting stent; LAD, left anterior descending; LCX, left circumflex; PCI, percutaneous coronary intervention; RCA, right coronary artery; SES, sirolimus-eluting stent; TIMI, thrombolysis in myocardial infarction.
The data are presented as No (%) or mean±standard deviation.
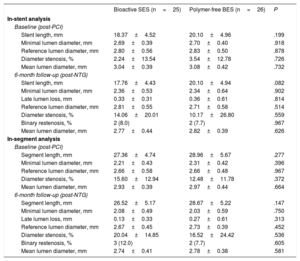
The angiographic analysis of the stent segment are shown in table 2. Postprocedural results were similar in the 2 groups. At 6 months, lumen loss was similar between the groups (0.33±0.31mm vs 0.36±0.61mm, respectively; P=.814). Binary restenosis was observed in 8.0% vs 7.7%, respectively (P=.967).
Quantitative coronary angiography analysis of the stent segment
| Bioactive SES (n=25) | Polymer-free BES (n=26) | P | |
|---|---|---|---|
| In-stent analysis | |||
| Baseline (post-PCI) | |||
| Stent length, mm | 18.37±4.52 | 20.10±4.96 | .199 |
| Minimal lumen diameter, mm | 2.69±0.39 | 2.70±0.40 | .918 |
| Reference lumen diameter, mm | 2.80±0.56 | 2.83±0.50 | .878 |
| Diameter stenosis, % | 2.24±13.54 | 3.54±12.78 | .726 |
| Mean lumen diameter, mm | 3.04±0.39 | 3.08±0.42 | .732 |
| 6-month follow-up (post-NTG) | |||
| Stent length, mm | 17.76±4.43 | 20.10±4.94 | .082 |
| Minimal lumen diameter, mm | 2.36±0.53 | 2.34±0.64 | .902 |
| Late lumen loss, mm | 0.33±0.31 | 0.36±0.61 | .814 |
| Reference lumen diameter, mm | 2.81±0.55 | 2.71±0.58 | .514 |
| Diameter stenosis, % | 14.06±20.01 | 10.17±26.80 | .559 |
| Binary restenosis, % | 2 (8.0) | 2 (7.7) | .967 |
| Mean lumen diameter, mm | 2.77±0.44 | 2.82±0.39 | .626 |
| In-segment analysis | |||
| Baseline (post-PCI) | |||
| Segment length, mm | 27.36±4.74 | 28.96±5.67 | .277 |
| Minimal lumen diameter, mm | 2.21±0.43 | 2.31±0.42 | .396 |
| Reference lumen diameter, mm | 2.66±0.58 | 2.66±0.48 | .967 |
| Diameter stenosis, % | 15.60±12.94 | 12.48±11.78 | .372 |
| Mean lumen diameter, mm | 2.93±0.39 | 2.97±0.44 | .664 |
| 6-month follow-up (post-NTG) | |||
| Segment length, mm | 26.52±5.17 | 28.67±5.22 | .147 |
| Minimal lumen diameter, mm | 2.08±0.49 | 2.03±0.59 | .750 |
| Late lumen loss, mm | 0.13±0.33 | 0.27±0.61 | .313 |
| Reference lumen diameter, mm | 2.67±0.45 | 2.73±0.39 | .452 |
| Diameter stenosis, % | 20.04±14.85 | 16.52±24.42 | .536 |
| Binary restenosis, % | 3 (12.0) | 2 (7.7) | .605 |
| Mean lumen diameter, mm | 2.74±0.41 | 2.78±0.38 | .581 |
BES, biolimus-eluting stent; NTG, nitroglycerin; PCI, percutaneous coronary intervention; SES, sirolimus-eluting stent.
Values are expressed as No. (%) or mean±standard deviation.
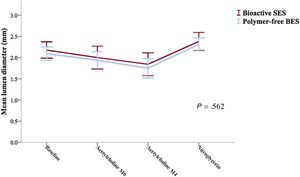
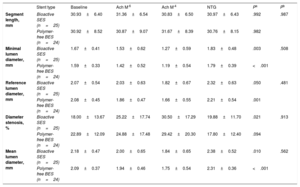
Vasomotor examination was performed in 49 patients (25 bioactive SES and 24 polymer-free BES). The endothelial-dependent and independent vasomotor responses at 6 months are shown in table 3. Both bioactive SES and polymer-free BES showed vasoconstriction to low dose (−8.3±20.1% vs−7.6±14.2%; P=.890) and high dose (−16.0±20.2% vs−16.1±21.6%; P=.983) of acetylcholine infusion. Endothelial dysfunction was frequent and was similarly observed in the 2 groups (64.0% vs 62.5%, respectively; P=.913). The mean lumen diameter changes of the distal coronary segment at 6 months is shown in figure 4.
Distal coronary segment vasomotor test results
| Stent type | Baseline | Ach M-6 | Ach M-4 | NTG | Pa | Pb | |
|---|---|---|---|---|---|---|---|
| Segment length, mm | Bioactive SES (n=25) | 30.93±6.40 | 31.36±6.54 | 30.83±6.50 | 30.97±6.43 | .992 | .987 |
| Polymer-free BES (n=24) | 30.92±8.52 | 30.87±9.07 | 31.67±8.39 | 30.76±8.15 | .982 | ||
| Minimal lumen diameter, mm | Bioactive SES (n=25) | 1.67±0.41 | 1.53±0.62 | 1.27±0.59 | 1.83±0.48 | .003 | .508 |
| Polymer-free BES (n=24) | 1.59±0.33 | 1.42±0.52 | 1.19±0.54 | 1.79±0.39 | <.001 | ||
| Reference lumen diameter, mm | Bioactive SES (n=25) | 2.07±0.54 | 2.03±0.63 | 1.82±0.67 | 2.32±0.63 | .050 | .481 |
| Polymer-free BES (n=24) | 2.08±0.45 | 1.86±0.47 | 1.66±0.55 | 2.21±0.54 | .001 | ||
| Diameter stenosis, % | Bioactive SES (n=25) | 18.00±13.67 | 25.22±17.74 | 30.50±17.29 | 19.88±11.70 | .021 | .913 |
| Polymer-free BES (n=24) | 22.89±12.09 | 24.88±17.48 | 29.42±20.30 | 17.80±12.40 | .094 | ||
| Mean lumen diameter, mm | Bioactive SES (n=25) | 2.18±0.47 | 2.00±0.65 | 1.84±0.65 | 2.38±0.52 | .010 | .562 |
| Polymer-free BES (n=24) | 2.09±0.37 | 1.94±0.46 | 1.75±0.54 | 2.31±0.36 | <.001 |
Ach, acetylcholine; BES, biolimus-eluting stent; NTG, nitroglycerin; SES, sirolimus-eluting stent.
Values are expressed as mean±standard deviation.
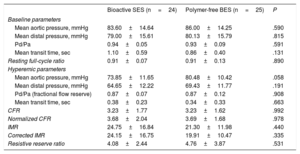
Microcirculatory function at 6 months is shown in table 4. Both bioactive SES and polymer-free BES had similar functional resting conditions. Hyperemic microcirculatory parameters were also similar between the groups and were within the normal reference values. Mean coronary flow reserve was 3.23±1.77 vs 3.23±1.62 (P=.992) and the index of microcirculatory resistance was 24.75±16.84 vs 21.30±11.98, respectively (P=.440).
Microcirculatory function results
| Bioactive SES (n=24) | Polymer-free BES (n=25) | P | |
|---|---|---|---|
| Baseline parameters | |||
| Mean aortic pressure, mmHg | 83.60±14.64 | 86.00±14.25 | .590 |
| Mean distal pressure, mmHg | 79.00±15.61 | 80.13±15.79 | .815 |
| Pd/Pa | 0.94±0.05 | 0.93±0.09 | .591 |
| Mean transit time, sec | 1.10±0.59 | 0.86±0.40 | .131 |
| Resting full-cycle ratio | 0.91±0.07 | 0.91±0.13 | .890 |
| Hyperemic parameters | |||
| Mean aortic pressure, mmHg | 73.85±11.65 | 80.48±10.42 | .058 |
| Mean distal pressure, mmHg | 64.65±12.22 | 69.43±11.77 | .191 |
| Pd/Pa (fractional flow reserve) | 0.87±0.07 | 0.87±0.12 | .908 |
| Mean transit time, sec | 0.38±0.23 | 0.34±0.33 | .663 |
| CFR | 3.23±1.77 | 3.23±1.62 | .992 |
| Normalized CFR | 3.68±2.04 | 3.69±1.68 | .978 |
| IMR | 24.75±16.84 | 21.30±11.98 | .440 |
| Corrected IMR | 24.15±16.75 | 19.91±10.47 | .335 |
| Resistive reserve ratio | 4.08±2.44 | 4.76±3.87 | .531 |
BES, biolimus-eluting stent; CFR, coronary flow reserve; IMR, index microcirculatory resistance; Pd/Pa, distal pressure/aortic pressure; SES, sirolimus-eluting stent.
Values are expressed as mean±standard deviation.
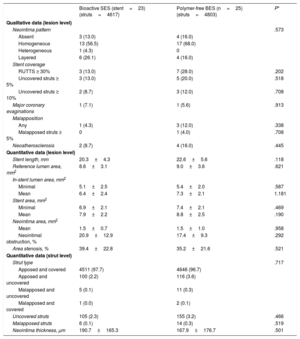
OCT was performed in 48 patients (23 bioactive SES and 25 polymer-free BES). OCT findings at 6 months are shown in table 5. All qualitative and quantitative OCT parameters were similar between the 2 groups and demonstrated a high grade of stent healing at 6 months. As examples, the percentage of uncovered struts (2.3% vs 3.2%; P=.466), patients with >5% of uncovered struts (13.0% vs 20.0%; P=.518) and major coronary evaginations (7.1% vs 5.6%; P=.708) were observed in only few cases. In-stent neoatherosclerosis was observed in 8.7% vs 16.0%, respectively (P=.445).
Optical coherence tomography findings
| Bioactive SES (stent=23) (struts=4617) | Polymer-free BES (n=25) (struts=4803) | P* | |
|---|---|---|---|
| Qualitative data (lesion level) | |||
| Neointima pattern | .573 | ||
| Absent | 3 (13.0) | 4 (16.0) | |
| Homogeneous | 13 (56.5) | 17 (68.0) | |
| Heterogeneous | 1 (4.3) | 0 | |
| Layered | 6 (26.1) | 4 (16.0) | |
| Stent coverage | |||
| RUTTS ≥ 30% | 3 (13.0) | 7 (28.0) | .202 |
| Uncovered struts ≥ 5% | 3 (13.0) | 5 (20.0) | .518 |
| Uncovered struts ≥ 10% | 2 (8.7) | 3 (12.0) | .708 |
| Major coronary evaginations | 1 (7.1) | 1 (5.6) | .913 |
| Malapposition | |||
| Any | 1 (4.3) | 3 (12.0) | .338 |
| Malapposed struts ≥ 5% | 0 | 1 (4.0) | .708 |
| Neoatherosclerosis | 2 (8.7) | 4 (16.0) | .445 |
| Quantitative data (lesion level) | |||
| Stent length, mm | 20.3±4.3 | 22.6±5.6 | .118 |
| Reference lumen area, mm2 | 8.6±3.1 | 9.0±3.6 | .621 |
| In-stent lumen area, mm2 | |||
| Minimal | 5.1±2.5 | 5.4±2.0 | .587 |
| Mean | 6.4±2.4 | 7.3±2.1 | 1.181 |
| Stent area, mm2 | |||
| Minimal | 6.9±2.1 | 7.4±2.1 | .469 |
| Mean | 7.9±2.2 | 8.8±2.5 | .190 |
| Neointima area, mm2 | |||
| Mean | 1.5±0.7 | 1.5±1.0 | .958 |
| Neointimal obstruction, % | 20.9±12.9 | 17.4±9.3 | .292 |
| Area stenosis, % | 39.4±22.8 | 35.2±21.6 | .521 |
| Quantitative data (strut level) | |||
| Strut type | .717 | ||
| Apposed and covered | 4511 (97.7) | 4646 (96.7) | |
| Apposed and uncovered | 100 (2.2) | 116 (3.6) | |
| Malapposed and uncovered | 5 (0.1) | 11 (0.3) | |
| Malapposed and covered | 1 (0.0) | 2 (0.1) | |
| Uncovered struts | 105 (2.3) | 155 (3.2) | .466 |
| Malapposed struts | 6 (0.1) | 14 (0.3) | .519 |
| Neointima thickness, μm | 190.7±165.3 | 167.9±176.7 | .501 |
BES, biolimus-eluting stent; RUTTS, ratio of uncovered to total stent struts; SES, sirolimus-eluting stent.
Values are expressed as No. (%) or mean±standard deviation.
* P value of strut level data has been estimated with generalized estimating equations taking into account the clustering nature of the optical coherence tomography data.
The main findings of the present study are: a) both bioactive SES (COMBO) and polymer-free BES (BioFreedom) showed mainly impaired endothelial-dependent vasomotor function and preserved endothelial-independent function of the distal epicardial IRA at 6 months; b) the microcirculatory function of the IRA had an almost preserved response to hyperemia without differences between the study groups; c) both bioactive SES and polymer-free BES showed an advanced healing state, as assessed by OCT, at 6 months.
The coronary endothelium is the natural monolayer cell barrier between blood and arterial wall. According to pathology studies, stent implantation causes denudation of the endothelium and provokes an inflammatory response. At the very early phase after BMS implantation (< 30 days), inflammatory cell infiltration, platelet aggregation and fibrin deposition are normally observed.8 Simultaneously, smooth muscle cell migration accompanied by extracellular matrix deposition often surround and cover the stent struts. For this reason, as assessed by OCT, BMS exhibit most of the stent struts apparently covered at 30 days. However, stent re-endothelialization after BMS implantation occurs 3 to 4 months later by proliferation and migration of surrounding vascular endothelial cells and by the adhesion and maturation of circulating endothelial progenitor cells.9 Unfortunately, OCT is unable to assess stent re-endothelialization because of the limited image resolution.
The healing process of durable and bioresorbable polymer DES is temporarily and substantially different than that observed with BMS. The antiproliferative drug inhibits smooth muscle cells and vascular endothelial cell migration at the very early phase, delaying the stent healing process even at very long-term follow-up.8 Polymer-free DES are designed to enhance the stent healing process by a fast release of the antiproliferative drug (most of the drug is released in <48hours). Therefore, the healing process of polymer-free BES is similar to that observed with BMS. One study using polymer-free BES showed almost complete stent coverage, as assessed by OCT, at 4 months.4 Bioactive SES take a further step by aiming to adhere circulating endothelial progenitor cell to the endoluminal stent surface, while the abluminal surface inhibits smooth muscle cell proliferation. Preclinical studies demonstrated almost complete re-endothelialization of the inner surface of the stent at 14 days.3 According to several large controlled studies with elective angiographic follow-up between 6 and 13 months, current types of durable polymer and bioresorbable polymer DES show <0.20mm angiographic lumen loss. In contrast, DES aimed to enhance stent healing, such as polymer-free BES and bioactive SES, show lumen loss >0.20mm. Although further investigations with large numbers of patients are required, DES aimed to enhance stent re-endothelialization seem to show a larger neointima response and restenosis than current iterations of durable polymer and bioresorbable polymer DES. Table 1 of the supplementary data summarizes the in-stent results of most of the studies using current generation DES with angiographic follow-up.
Several randomized trials have shown differences regarding the endothelial function of different stent types in non-STEMI patients. It is commonly assumed that BMS mostly preserve the normal endothelial function of the distal coronary segment (vasodilatation) when the stent has fulfilled the healing process (approximately at 6 months). Mean lumen diameter changes to endothelial-dependent stimuli of the distal coronary segment treated with BMS are reported to be between−2.5% to+8.6%.10–12 However, the small numbers of patients, use of different vasomotor tests (such as rapid pacing, supine exercise, or acetylcholine infusion) and different methods used for quantitative coronary angiography analysis warrant careful interpretation of these data. First-generation durable polymer DES are commonly accepted to lead to the worst endothelial function (vasoconstriction between 23.6% to 3.4%)10,11 and second-generation durable polymer DES (vasoconstriction between 9.4% to 3.1%) and bioresorbable polymer DES (vasoconstriction around 8.6%) show a certain degree of endothelial dysfunction.1,6,12,13
Endothelial dysfunction seems more intense in STEMI patients.14 First, STEMI patients show systemic inflammation and microvascular dysfunction of several organs and coronary vessels affecting the normal epicardial endothelial function.2 Second, stent implantation modifies the vessel geometry and endothelial shear stress forces, especially in the contiguous stent segments. Coronary segments with low endothelial shear stress, such as stent edge segments, show larger degree of endothelial dysfunction than segments with normal or high endothelial shear stress.15 Finally, stent implantation denudates the coronary endothelium and consequently, endothelial dysfunction is generally observed in distal coronary segments immediately after stent implantation.16 DES are designed to delay stent healing and reendothelization and are associated with a larger amount of malapposed and protruding stent struts than BMS. Malapposed and protruding stent struts cause flow disturbances similar to those observed in segments with low endothelial shear stress.15 Notably, STEMI lesions treated with DES show worse stent healing than non-STEMI lesions.17,18 In addition, the direct drug action of current DES, inflammatory reaction to different stent polymers and the degree of stent re-endothelialization have been pointed out as potential mechanisms of endothelial dysfunction.15
According to the few studies performed of endothelial function in STEMI patients, distal segments of the IRA treated with BMS showed 7.9% vasoconstriction to intracoronary acetylcholine at 6 months19; bioresorbable polymer SES (Orsiro, Biotronik, Switzerland) showed 18.1±15.4% vasoconstriction at 1 year20; and durable polymer everolimus-eluting stent (XIENCE, Abbott, United States) showed 8.7±14.8% vasoconstriction at 3 years.5 Therefore, taking into account the limitations of comparing different studies with different angiographic follow-ups, bioactive SES (16.0±20.2% vasoconstriction) and polymer-free BES (16.1±21.6% vasoconstriction) seem to have a similar vasomotor response at 6 months as bioresorbable polymer SES at 1 year, but worse endothelial function than durable polymer everolimus-eluting stent at 3 years. The endothelial function of all 4 DES in STEMI patients is summarized in table 2 of the supplementary data.
LimitationsFirst, the present study has no sample size calculation. Therefore, all comparisons between devices are merely hypothesis generating. Second, this study recruited <10% of patients undergoing primary PCI during the study period and therefore the sample is not representative of an all-comers STEMI population. Third, vasomotor examination was performed at 6 months. Although both study devices have theoretically fulfilled the healing process according to preclinical studies, it is possible that human models may have slower healing and therefore the endothelial function of both devices could be better at longer follow-up. Finally, due to the methodology used in the present study, epicardial and microvascular vasospastic angina were not evaluated. To prevent complications related to provocative vasospastic tests, such as occlusion of proximal coronary segments, it was decided to selectively infuse intracoronary acetylcholine via a microcatheter.
CONCLUSIONSIRA treated either with bioactive SES (COMBO) or polymer-free BES (BioFreedom) showed a similar epicardial distal endothelial vasomotor response to acetylcholine infusion and similar microcirculatory response to hyperemia at 6 months. Endothelial dysfunction was frequently observed despite preserved functional microcirculatory parameters of the IRA and almost complete stent coverage, as assessed by OCT at 6 months. Larger studies are needed to assess the role of endothelial dysfunction in STEMI patients.
FUNDINGThis study was funded by OrbusNeich.
CONFLICTS OF INTERESTJ. Gómez-Lara received fees from BARCICORE-Lab. S. Brugaletta reports consultant fees from Boston Scientific and iVascular. M. Sabaté reports consultant fees from Abbott Vascular and iVascular. The other authors declare no conflicts of interest.
- -
Distal segments to coronary stents show a different vasomotor response to endothelial-dependent stimuli. In general, BMS show better endothelial function than DES, which has been attributed to the better stent healing and re-endothelialization of BMS. Moreover, STEMI patients show worse stent healing of current DES than stents implanted in other clinical scenarios. New-generation DES, such as polymer-free and bioactive DES, are designed with the aim of enhancing stent coverage and re-endothelialization. However, the endothelial function of distal coronary segments treated with those stents in STEMI patients is largely unknown.
- -
This is the first study investigating the coronary function of new stent technologies aiming to promote stent re-endothelialization in STEMI patients. Although minor differences between stent technologies can be hypothesized, the endothelial function observed in STEMI patients was severely impaired and may have multiple causes. Moreover, endothelial dysfunction was observed despite optimal stent healing and microvascular function. Further investigations are required to address the role of stent-related endothelial dysfunction in STEMI patients.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.01.007