Bioresorbable vascular scaffolds (BVS), introduced to clinical practice some 10 years ago, represented a real conceptual revolution in interventional cardiology.1–4 The possibility of obtaining the same results as with the latest generation of conventional metallic drug-eluting stents (DES), but with a technology that left “no trace” of the intervention in the vessel wall, represented an interesting paradigm shift that seemed difficult to resist.2–4 Their structural platform guaranteed satisfactory support of the vascular wall, avoiding early elastic recoil, and excellent immediate angiographic results (stent-like), in addition to their potent antiproliferative drug that effectively inhibited neointimal growth.2–5 However, the major attraction of BVS was that, once they had completed the function they had been designed for, both their structural elements and the polymer required as the medium for the drug disappeared completely from the coronary wall.2–5 Some initial studies confirmed that the entire process culminating with the complete dissolution of the device, took about 3 years to complete.2–5
As such, BVDs were able to overcome some of the limitations still present in latest -generation DES, attributed mainly to the indefinite persistence of foreign objects in the vascular wall. Indeed, in some patients this appeared to represent a real Sword of Damocles, with a minimal, but real and persistent risk of very late thrombosis or restenosis (more than 1 year after implantation) of the DES.6,7
In contrast, once the BVS had completely disappeared, the artery was then “free” from the restraint of the permanent metallic elements of the DES and recovered its ability to acutely respond to different vascular physiological stimuli (vasoconstriction and vasodilation) and, in the longer term, to the potential favorable effects of shear stress both on progression/regression of the atheromatous plaque and its phenotype and on the phenomena of late vascular remodelling.2–5 Some rather provocative data, from the current perspective, even suggested the possibility that BVS may induce volume regression of the underlying plaque, facilitate passivation of potentially vulnerable plaques (providing a new fibrous covering to the fine capsule of the fibroatheroma) and even achieve an additional reduction in angina symptoms in some patients (this phenomenon was never well explained), attributed to a more complete recovery of endothelial function in the entire vessel.2–5 In the initial phase of this wave of optimism, the boldest proponents even suggested changing the common language used in interventional cardiology with the introduction of much more seductive, attractive terms such as complete vascular “repair” and “restoration”.
Furthermore, the resorption of the BVS meant freedom of the side branches that were caged by the device, and the disappearance of its inconveniently protruding parts (in ostial lesions and bifurcations) or underexpanded parts (in calcified lesions), thus resolving the problem of late malapposition (persistent or acquired), and facilitating future treatment options, either by further percutaneous interventions or surgical anastomoses in coronary segments now completely free from any device (figure 1).2–5
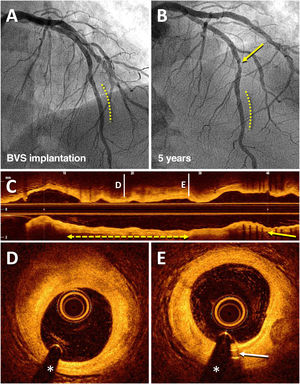
Angiographic result on optical coherence tomography of a bioresorbable vascular scaffold (BVS) implanted 5 years prior in the left anterior descending artery (LAD). An 83-year-old man who, in 2013, for stable angina, received a BVS (Absorb) (3 × 18mm) in a lesion in the distal LAD (dashed line), with an excellent result (A). In 2018, coronary angiography was repeated due to stable angina, which showed the good result of the BVS (dashed line) and a de novo lesion in the mid segment of LAD (arrow) that was treated with a drug-eluting stent (B). Subsequent imaging with optical coherence tomography showed an excellent result with the recently implanted drug-eluting stent (C; arrow) and the complete disappearance of the BVS (C-E); in that coronary segment, there was only an unobstructed fibrous plaque (D; dashed line with arrows) and the residual radiopaque markers (platinum) at the ends of the device (E; arrow).
So, what happened? Why are we talking in the past tense? We now know that, despite the excellent initial outcomes demonstrated in various observational studies and controlled clinical trials, rigorous follow-up in the very long term and new meta-analyses (including a much greater number of patients) have reliably demonstrated that BVS were inferior to latest-generation DES in terms of target lesion failure (due to significantly higher incidences of restenosis and device thrombosis).8 This resulted in the most-used and most-studied polymer-based BVS (based on polylactic acid degradation via the Krebs’ cycle), Absorb (Abbott Vascular, USA), finally being taken off the market. Although other BVS are available that may offer better results, the latest European revascularization guidelines are absolutely clear in their recommendations, stating that, at present, the use of BVS is not indicated (class III A) in routine clinical practice.9 Although a class effect of BVS had not been demonstrated, this caution seems not only justified but necessary.
Why did this happen? Were we—once again—too optimistic? Did we try—once more—to run before we could walk? There have always been sceptics and critics of this new technology. The structural elements of the scaffold required a larger caliber than those used in latest-generation DES (increasingly finer and with better clinical outcomes) to obtain the same radial force and guarantee the essential functions of vascular wall support.2–5,7,10 In addition, due to the type of material used, they were less flexible, less navigable through tortuous coronary segments, their resistance to breaking (for example, in the case of over-dilatation) was much lower, and fewer sizes were available (in terms of diameter and length). In highly calcified lesions, cases of early device collapse were observed. The thickest struts also tended to compromise side branches to a greater extent and treatment of these could damage the scaffold platform. Therefore, initially, it was strongly recommended that they be used with caution, and restricted to patients without complex anatomies.2–5,7,10 Later, when there were some difficulties in achieving good implantation in more unfavorable lesions, it was advised to optimize them by routine predilatation and postdilatation and by guiding the implantation either with quantitative measurements on coronary angiography, or with intracoronary diagnostic techniques.3–5 All of this highlighted that BVS could not compete against the newer DES, at least in everyday clinical practice.8 In addition, subsequent data demonstrated the persistence of structural elements in the vessel wall for longer than expected. This was attributed to their degradation in coronary segments with advanced atherosclerosis possibly being slower than that demonstrated in initial in vivo studies or in patients with favorable lesions. These findings also helped explain some disappointing results in controlled studies on the recovery of arterial dynamics.5 However, much more worrying still was the finding that, occasionally, the degradation of the device was not as clean and complete as previously thought. In some patients, the BVS degradation process was associated with nonhomogeneous loss of structural integrity that led to an inadequate lack of wall support or the appearance of partially-resorbed scaffold struts within the vascular lumen and, much worse still, which was associated in some patients with clinical presentations such as restenosis or very late stent thrombosis.10–12 The potential mechanisms involved in late BVS failure have been described in detail.10–12 Finally, the worse overall target lesion results, confirmed in the aforementioned meta-analyses, represented the final nail in the coffin (requiescant in pace) for these first-generation BVS.8
Nevertheless, should BVS remain buried forever? Might they have some advantages over DES in specific clinical or anatomical contexts that, regrettably, we have not been able to identify? What about newer generations of BVS, with much more advanced technology? If, based on what we have learned so far, we managed to solve the initial difficulties, could they go from being an attractive treatment strategy to a clinically superior one? Perhaps in very select patients? What really happens in these treated coronary segments with excellent late results, once the device disappears completely? (figure 1) Will they really remain immune from very late restenosis and thrombosis?
In a recently-published article in Revista Española de Cardiología, Wiebe et al.13 analyzed the very late clinical outcomes with BVS. The authors studied the 5-year follow-up outcomes from the prospective registry ISAR-ABSORB, which included consecutive, unselected patients (within routine clinical practice) treated with polymeric everolimus-eluting BVS (Absorb) in 2 large German hospitals. The analysis included 419 patients treated with BVS and a total of 527 lesions. The indication for treatment was acute coronary syndrome in 40% of the patients; one third had diabetes, one quarter had renal failure, and half of the lesions were considered angiographically complex (mean length, 16mm). The primary endpoint (a composite of death, myocardial infarction and revascularization of the target lesion) occurred in one third of the patients (33.1%) mainly due to the need for further revascularization of the target lesion (20.3%). In addition, 4.7% of the patients had device thrombosis, most of them after the first 2 years of follow-up. None of the patients with thrombosis were taking dual antiplatelet therapy, and in the patients with an available optical coherence tomography scan, it showed disruption of the scaffold, malapposition, or aneurysmal areas.13
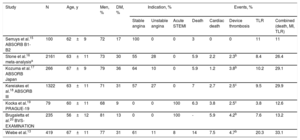
Until now, the available very long-term follow-up data on BVS came from only the most selected patients included in controlled studies, mostly randomized clinical trials (table 1). Such patients generally have much more favorable clinical and anatomical characteristics than the less selected patients who are treated in day-to-day clinical practice. We would even have to accept that the patients who are chosen for treatment with BVS may already have certain anatomical characteristics that are more favorable (selection bias) than patients treated with DES, due to the aforementioned navigability limitations inherent to these devices. The initial data at 2 years from this German registry has already been published,14 and the present article reports the follow-up of these patients at 5 years. These results generate some potentially interesting reflections.
Studies of bioresorbable vascular scaffolds with 5-year clinical outcomes r
| Study | N | Age, y | Men, % | DM, % | Indication, % | Events, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stable angina | Unstable angina | Acute STEMI | Death | Cardiac death | Device thrombosis | TLR | Combined (death, MI, TLR) | |||||
| Serruys et al.15 ABSORB B1-B2 | 100 | 62±9 | 72 | 17 | 100 | 0 | 0 | 3 | 0 | 0 | 11 | 11 |
| Stone et al.16 meta-analysisa | 2161 | 63±11 | 73 | 30 | 55 | 28 | 0 | 5.9 | 2.2 | 2.3b | 8.4 | 26.4 |
| Kozuma et al.17 ABSORB Japan | 266 | 67±9 | 79 | 36 | 64 | 10 | 0 | 5.9 | 1.2 | 3.8b | 10.2 | 29.1 |
| Kereiakes et al.18 ABSORB III | 1322 | 63±11 | 71 | 31 | 57 | 27 | 0 | 7 | 2.7 | 2.5c | 9.5 | 29.9 |
| Kocka et al.19 PRAGUE-19 | 79 | 60±11 | 68 | 9 | 0 | 0 | 100 | 6.3 | 3.8 | 2.5c | 3.8 | 12.6 |
| Brugaletta et al.20 BVS-EXAMINATION | 235 | 56±12 | 81 | 13 | 0 | 0 | 100 | - | 5.9 | 4.2b | 7.6 | 13.2 |
| Wiebe et al.13 | 419 | 67±11 | 77 | 31 | 61 | 11 | 8 | 14 | 7.5 | 4.7b | 20.3 | 33.1 |
DM, diabetes mellitus; MI, myocardial infarction; STEMI, ST-elevation myocardial infarction; TLR, target lesion revascularization.
All studies involved the Absorb bioresorbable vascular scaffold.
In the study,13 the authors recommend routine predilatation of lesions before BVS implantation, but postdilatation was left to the operator's discretion and, in fact, was not done in a third of cases. At the beginning of the study, the researchers did not know, at least not as clearly as now, the importance of careful postdilatation of BVS to optimize the results and of guiding the implant with intracoronary diagnostic techniques, and these were only used anecdotally. We do not know if the long-term clinical outcomes were worse in patients without postdilatation (although this was not seen on multivariate analysis), so we could speculate on the possibility that the very late clinical outcomes could have been better if all the patients had benefitted from postdilatation and intracoronary image guidance. Obviously, this would be less representative of everyday clinical practice, which is what this registry was attempting to analyze.
Although these patients represent real-world practice and were practically unselected, the high rate of events, especially late thrombosis, were similar or slightly higher than those found in the few published controlled studies with long-term follow-up of this device (table 1).15–20 In all the studies, a low but constant rate of events related to the treated segment was maintained after 2 to 3 years after BVS implantation.
In this study, the authors did not plan or systematically study the prescription of or adherence to long-term antiplatelet therapy (single, dual with clopidogrel, or dual with prasugrel or ticagrelor), so there is also the possibility that prolonged antiplatelet therapy, adjusted to the patient's ischemic risks (multivessel, overlapping BVS) and hemorrhagic risks would have achieved better clinical outcomes.
In addition, all the patients in the study were recommended to undergo follow-up angiography (6-8 months); in the end, this was done in 71% of patients. This approach, firstly, does not reflect routine clinical practice and could have led to another selection bias and secondly, always increases the number of revascularizations done during follow-up (oculostenotic reflex) even if clinical criteria (angina or ischemia) are required for their indication. The existence of this phenomenon can be seen clearly on examining the Kaplan-Meier curves, for both the primary outcome variable and for revascularization-free survival.
However, without doubt, the most interesting finding of this study is that it demonstrated that long-term adverse events continued to occur, in a constant and stable way, without plateauing. Again, this can be seen on the actuarial survival curves and, even more clearly, with the specific analysis done after 2 years.
It is possible that we did not know how to identify the patients that could benefit most from these attractive devices. It is also possible that we have not taken into account the special care that BVS require for optimal implantation (routine predilatation and postdilatation, intravascular imaging guidance). While all that could be true, what is indisputable is that the results obtained with the currently-available BVS cannot compete with those from latest-generation DES, not only in controlled trials but also in real-life clinical practice. The new data from the study by Wiebe et al.13 and other controlled studies with 5-year follow-up15–18 appear to indicate that coronary segments treated with BVS are not free from very-long-term complications. These could be secondary to a delayed or deficient resorption of the device that would allow these segments to remain vulnerable in the very long term. The other possibility is that in the coronary segment where the BVS is implanted, there may be ongoing disease, despite the complete dissolution of the device, leading to restenosis or neoatherosclerosis (or reactivation of the underlying atherosclerosis, if preferred), and requiring further revascularization, or forming the substrate for an atherothrombotic complication causing an acute myocardial infarct. Both mechanisms could affect the reported outcomes. As such, the Sword of Damocles will remain present in the diseased vessel wall and the phenomenon of complete vascular repair or restoration would be, in reality, a myth. Will BVS be “brought back to life” with the help of new biotechnological advances? It is entirely possible, but it will not be easy for us to get over the hard lessons that we have learned along the way. For this therapeutic line to continue developing, it would need to demonstrate a clear clinical benefit in the long term, at least for certain specific indications. A few years ago we reminded readers that a rigorous and critical evaluation of the results obtained with any new coronary treatment must be the cornerstone of development in interventional cardiology, as the treatment of our patients cannot be based on expectations alone, no matter how attractive they may be.10
CONFLICTS OF INTERESTNone declared.