Long-term outcomes of unselected patients treated with bioresorbable vascular scaffold (BVS) implantation are lacking, especially for the period after complete dissolution of the BVS. This study sought to evaluate 5-year outcomes in patients treated with BVS in routine practice.
MethodsConsecutive patients who underwent implantation of everolimus-eluting BVS during routine clinical practice at 2 high-volume centres in Germany were studied. The patients were followed-up for up to 5 years. The primary endpoints of interest were the composite of death, myocardial infarction and target lesion revascularization, as well as definite scaffold thrombosis.
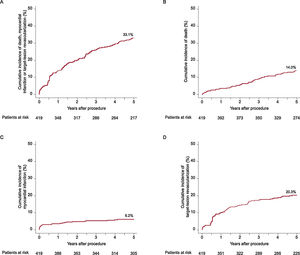
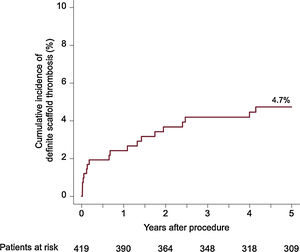
ResultsA total of 419 patients (mean age 66.6 ± 10.9 years; 31.5% had diabetes) were included, of whom 38.9% presented with an acute coronary syndrome. Of the 527 lesions treated, 49.0% were classified as complex and 13.1% were bifurcation lesions. At 5 years, the composite clinical endpoint occurred in 33.1% of patients and definite scaffold thrombosis occurred in 4.7%. Most definite scaffold thrombosis occurred within 2 years after BVS implantation.
ConclusionsIn patients treated with BVS implantation in routine clinical practice the rates of adverse clinical events at 5 years were high, including a considerable incidence of scaffold thrombosis.
Keywords
Bioresorbable vascular scaffolds (BVS) were developed to address the limitations of metallic drug-eluting stent (DES) technology.1 Due to the self-degrading nature of the scaffold backbone within approximately 3 years,2 it was hypothesized that the risk of late adverse events seen with conventional DES might be ameliorated.3 Additional proposed advantages of BVS over metallic DES included late luminal enlargement and restoration of vasomotion in the stented vessel.4 Although the initial results of BVS treatment in small observational studies of patients with noncomplex lesions were encouraging, subsequent data raised concerns about device performance. In several randomized trials comparing everolimus-eluting BVS (Absorb, Abbott Vascular) with DES, a trend toward increased rates of stent thrombosis in the BVS group was observed at 1 year.5 The explanations proposed included inappropriate lesion selection and implantation technique.6 Meta-analysis of longer-term data from several randomized trials found an increased risk of stent thrombosis at a median follow-up of 26.6 months, with the risk being particularly high beyond 1 year, a finding that has been confirmed in longer-term follow-up of randomized trials.7
The observed performance of BVS in randomized controlled trials occurs in the setting of strict inclusion and exclusion criteria, with underrepresentation of many clinical and anatomical scenarios. Indeed, postmarket surveillance of approved devices in clinical registries plays an important part in medical device evaluation and the availability of long-term outcome data in patients treated with BVS as part of routine clinical use is an important unmet need. The present study aimed to address this knowledge gap by evaluating 5-year clinical outcomes of patients who underwent BVS implantation in routine practice, when the BVS is thought to be completely dissolved.
METHODSStudy design and patient selectionThe ISAR Absorb Registry is a prospective, nonrandomized, observational study, conducted at 2 high-volume centres in Germany. Between September 2012 and June 2014, consecutive symptomatic patients with de novo lesions undergoing implantation of everolimus-eluting BVS were enrolled. All patients provided written consent for collection of clinical data at the time of hospital admission. This investigation was performed in accordance with the Declaration of Helsinki and was approved by the local ethics committee. Further details of the study design have been published previously.8
Study procedure and medicationsPercutaneous coronary intervention was performed according to current recommendations at the time of implantation.9,10 The BVS (Absorb, Abbott Vascular) has a poly-L-lactic acid backbone and its coating consists of poly-D-L-lactic acid and everolimus. Predilatation was recommended for all lesions and the decision to perform postdilatation was left to the discretion of the implanting physician. Procedural success was defined as residual stenosis<30% and thrombolysis in myocardial infarction 3 flow. Periprocedural unfractionated heparin or bivalirudin was administered in all patients. A loading dose of aspirin and an adenosine-diphosphate (ADP) receptor antagonist was given, followed by aspirin indefinitely and a minimum of 12 months of the selected ADP receptor antagonist, depending on the clinical presentation.10 In patients receiving concomitant oral anticoagulation, the therapeutic regimen and duration of antiplatelet therapy was prescribed on an individual basis at the operator's discretion. The duration of triple therapy was limited to 6 months.
Follow-upDuring the hospital stay, electrocardiogram recordings and lab tests were performed daily until discharge. Routine angiographic follow-up was recommended for all patients at 6 to 8 months and further clinical telephone follow-ups were scheduled at 1 and 12 months and annually thereafter up to 5 years. The primary endpoint of interest was the composite of death, myocardial infarction and ischemia-driven target lesion revascularization (TLR). Secondary endpoints included the individual components of the primary endpoint and definite stent thrombosis according to Academic Research Consortium criteria.11 All deaths were classified as cardiac death in the absence of a clear noncardiovascular cause.
Quantitative coronary angiography analysisAn automated edge-detection system (CMS version 7.1, Medis Medical Imaging Systems) was used for the offline quantitative coronary angiography analysis of the index and follow-up angiogram. The major parameters of interest included percentage diameter stenosis, in-segment binary restenosis, and in-stent late luminal loss, which was defined as the difference between minimal lumen diameter postimplantation and minimal lumen diameter at angiographic follow-up. Bifurcation lesions were defined as a lesion occurring at or adjacent to a significant branch of a major coronary artery.
Statistical analysisCategorical variables are presented as counts and percentages and continuous variables are presented as median with interquartile range or mean with standard deviation. The Kaplan-Meier method was used to calculate event rates. Cumulative incidence functions were computed for endpoints other than death to account for competing risks. The Least Absolute Shrinkage and Selection Operator (LASSO) regression method provided in the R-package “glmnet” was used for variable selection for the multivariable model after entering all baseline and procedural characteristics. A Cox proportional model was then applied after entering a cluster term to account for the frequent presence of multiple treated lesions in the same patient. A P-value of <.05 was considered significant.
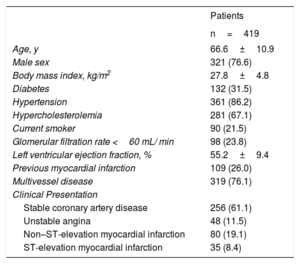
RESULTSBaseline characteristicsDuring the enrolment period, a total of 419 patients with a mean age of 66.6±10.9 years were analyzed. Most of the patients (76.6%) were male, 31.5% had a history of diabetes, and 38.9% presented with acute coronary syndrome. Details of baseline patient characteristics are displayed in table 1.
Baseline characteristics
| Patients | |
|---|---|
| n=419 | |
| Age, y | 66.6±10.9 |
| Male sex | 321 (76.6) |
| Body mass index, kg/m2 | 27.8±4.8 |
| Diabetes | 132 (31.5) |
| Hypertension | 361 (86.2) |
| Hypercholesterolemia | 281 (67.1) |
| Current smoker | 90 (21.5) |
| Glomerular filtration rate <60 mL/ min | 98 (23.8) |
| Left ventricular ejection fraction, % | 55.2±9.4 |
| Previous myocardial infarction | 109 (26.0) |
| Multivessel disease | 319 (76.1) |
| Clinical Presentation | |
| Stable coronary artery disease | 256 (61.1) |
| Unstable angina | 48 (11.5) |
| Non–ST-elevation myocardial infarction | 80 (19.1) |
| ST-elevation myocardial infarction | 35 (8.4) |
The data are expressed as No. (%) or mean±standard deviation.
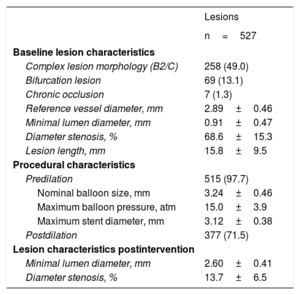
A total of 527 lesions were treated, of which 49.0% were classified as American College of Cardiology/American Heart Association B2/C lesion morphology and 13.1% were bifurcation lesions. Predilatation was performed in 97.7% of lesions and postdilatation was performed in 71.5% of lesions. A mean of 1.2±0.4 BVS per lesion with a mean length of 26.9±13.2 mm were implanted. A BVS overlap was present in 75 (17.9%) patients, in 41 patients due to the treatment of long lesions and in 34 patients due to additional BVS implantation because of dissection. Optical coherence tomography was used in 4.1% during implantation. Procedural success was achieved in 96.8% of patients. Details of angiographic and procedural characteristics are displayed in table 2. Most patients (95.5%) were discharged on aspirin and all patients received an ADP receptor antagonist, while 14.1% were discharged on oral anticoagulation.
Angiographic and procedural results
| Lesions | |
|---|---|
| n=527 | |
| Baseline lesion characteristics | |
| Complex lesion morphology (B2/C) | 258 (49.0) |
| Bifurcation lesion | 69 (13.1) |
| Chronic occlusion | 7 (1.3) |
| Reference vessel diameter, mm | 2.89±0.46 |
| Minimal lumen diameter, mm | 0.91±0.47 |
| Diameter stenosis, % | 68.6±15.3 |
| Lesion length, mm | 15.8±9.5 |
| Procedural characteristics | |
| Predilation | 515 (97.7) |
| Nominal balloon size, mm | 3.24±0.46 |
| Maximum balloon pressure, atm | 15.0±3.9 |
| Maximum stent diameter, mm | 3.12±0.38 |
| Postdilation | 377 (71.5) |
| Lesion characteristics postintervention | |
| Minimal lumen diameter, mm | 2.60±0.41 |
| Diameter stenosis, % | 13.7±6.5 |
The data are expressed as No. (%) or mean±standard deviation.
Quantitative coronary angiography analysis after 6 to 8 months was available for 71.0% (374/527) of lesions. In-stent late lumen loss was 0.27±0.51 mm and in-segment diameter stenosis was 27.7±16.1%. The rate of binary restenosis was 8.0%.
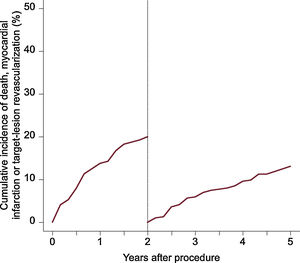
The median follow-up duration was 4.9 years. The 5-year rate of the composite endpoint of death, myocardial infarction and TLR was 33.1%, 14.0% of patients died, cardiac mortality was 7.5%, 6.2% experienced a myocardial infarction, and 20.3% underwent TLR. A total of 81 patients underwent TLR, of which 81.4% were symptomatic and 7.4% had evidence of ischemia. Definite scaffold thrombosis was observed in 4.7% of patients. A clustering of definite scaffold thrombosis was seen within the first 3 months after BVS implantation: of these 8 cases, the underlaying lesion was considered to be complex in 5 cases, postdilation was performed in 3 cases, and a 2.5-mm BVS was implanted in 1 case at the index procedure. At the time of scaffold thrombosis, all patients except 1 were on dual antiplatelet therapy. A total of 9 very late definite scaffold thrombosis were observed between 1 and 5 years after implantation. None of these patients were on dual antiplatelet therapy at the time of event. In 4 of these patients, optical coherence tomography imaging was performed, showing scaffold discontinuation with malapposed struts in 3 cases, of which 1 also had evidence of restenosis and a tissue bridge possibly related to chronic malapposition. In 1 patient an aneurysm in the BVS region was observed. Details of clinical outcomes are displayed in table 3 and time-to-event curves are displayed in figure 1 and figure 2. A landmark analysis of the composite endpoint is shown in figure 3.
Clinical outcomes until 5 years shown as Kaplan-Meier estimates
| Patients | |||||
|---|---|---|---|---|---|
| n=419 | 1 year | 2 years | 3 years | 4 years | 5 years |
| All-cause death | 3.6 | 5.6 | 9.5 | 11.9 | 14.0 |
| Cardiac death | 2.2 | 2.9 | 5.1 | 5.9 | 7.5 |
| Myocardial infarction | 3.6 | 4.9 | 5.4 | 5.9 | 6.2 |
| Death or myocardial infarction | 6.5 | 9.5 | 13.6 | 16.0 | 18.4 |
| Definite stent thrombosis | 2.4 | 3.7 | 4.2 | 4.4 | 4.7 |
| Target lesion revascularization | 9.9 | 14.4 | 17.2 | 18.8 | 20.3 |
| Composite of death, myocardial infarction, target lesion revascularization | 14.0 | 20.0 | 26.5 | 29.6 | 33.1 |
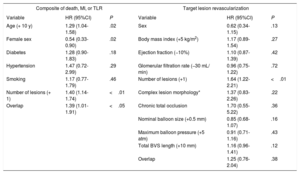
In the multivariate analysis, female sex (hazard ratio [HR], 0.54; 95% confidence interval [CI], 0.33-0.90; P=.02), a higher age (+ 10 years; HR, 1.29; 95%CI, 1.04-1.58; P=.02), the number of lesions treated (HR, 1.40; 95%CI, 1.14-1.74; P <.01) and BVS overlap (HR, 1.39; 95%CI, 1.01-1.91; P <.05) had an impact on the primary composite endpoint of death, myocardial infarction and TLR, whereas female sex was protective. The only independent predictor of TLR was the number of lesions treated (HR, 1.64; 95%CI, 1.22-2.21; P <.01). Further details of the multivariable analysis are provided in table 4.
Multivariate analysis of predictors of the composite primary endpoint and target lesion revascularization
| Composite of death, MI, or TLR | Target lesion revascularization | ||||
|---|---|---|---|---|---|
| Variable | HR (95%CI) | P | Variable | HR (95%CI) | P |
| Age (+ 10 y) | 1.29 (1.04-1.58) | .02 | Sex | 0.62 (0.34-1.15) | .13 |
| Female sex | 0.54 (0.33-0.90) | .02 | Body mass index (+5 kg/m2) | 1.17 (0.89-1.54) | .27 |
| Diabetes | 1.28 (0.90-1.83) | .18 | Ejection fraction (−10%) | 1.10 (0.87-1.39) | .42 |
| Hypertension | 1.47 (0.72-2.99) | .29 | Glomerular filtration rate (−30 mL/ min) | 0.96 (0.75-1.22) | .72 |
| Smoking | 1.17 (0.77-1.79) | .46 | Number of lesions (+1) | 1.64 (1.22-2.21) | <.01 |
| Number of lesions (+ 1) | 1.40 (1.14-1.74) | <.01 | Complex lesion morphology* | 1.37 (0.83-2.26) | .22 |
| Overlap | 1.39 (1.01-1.91) | <.05 | Chronic total occlusion | 1.70 (0.55-5.22) | .36 |
| Nominal balloon size (+0.5 mm) | 0.85 (0.68-1.07) | .16 | |||
| Maximum balloon pressure (+5 atm) | 0.91 (0.71-1.16) | .43 | |||
| Total BVS length (+10 mm) | 1.16 (0.96-1.41) | .12 | |||
| Overlap | 1.25 (0.76-2.04) | .38 | |||
95%CI, 95% confidence interval; BVS, bioresorbable scaffold; HR, hazard ratio; MI, myocardial infarction; TLR, target lesion revascularization.
The present study evaluates the long-term clinical results of patients undergoing BVS implantation without restrictions in routine clinical practice. The main findings are as follows: a) clinical event rates at 5 years were considerable despite generally satisfactory angiographic results at 6 to 8 months; b) the high rate of the composite endpoint of interest—death, myocardial infarction and TLR—at 5 years was largely driven by a high rate of TLR; c) the occurrence of the primary endpoint was significantly impacted by more advanced age, female sex, the number of treated lesions, and BVS overlap; and d) the high rate of definite scaffold thrombosis observed is in line with that in other BVS studies.
BVS were designed to ameliorate the inherent risk of late and very late failure with conventional stent implantation. After transient mechanical support of the vessel, the scaffold degradation process takes approximately 36 months until the BVS has fully dissolved.2 It was expected that the benefit of BVS over DES would become apparent during or after full resorption of the device. Initial results from a small, nonrandomized study with mainly simple lesions were promising. Clinical event rates were low and favorable effects such as late luminal enlargement and restoration of vasomotion were seen at 5 years.12,13 However, these positive results were not reproduced in large-scale randomized trials and neither are they reflected in the results of the present registry analysis. The overall disappointing results from randomized studies have led to severe safety concerns regarding BVS and therefore this investigated BVS was finally taken from the market. Consequently, the use of any BVS technology in daily practice received a class III recommendation, meaning that BVS should not be used outside the setting of clinical studies.14 Although the designs and compositions of other types of BVS vary significantly, the body of evidence regarding these other BVS types is very poor, supporting this recommendation and emphasizing the desperate need for further research. Nevertheless, data from long-term clinical follow-up studies including ours remain important to capture information on the clinical efficacy during and after the dissolution process.
In our study, the overall rate of major adverse cardiac events during follow-up was higher than would be expected from comparator datasets of patients treated with conventional DES. Indeed, a recent analysis of patients enrolled in 2 real-world clinical trials at the same centers that contributed to the present analysis showed overall rates of a similar patient-oriented composite endpoint of around 28% at 5 years compared with 33.1% in the present report.15 Moreover, looking at the individual components of the composite endpoint, 2 observations might be made. First, the event rate was driven mainly by TLR. Interestingly and contrary to the expectations of this technology, the rate of TLR continued to increase beyond 1 year and doubled to around 20% between 12 and 60 months. At the same time, more than two thirds of the patients underwent surveillance angiography after 6 to 8 months and this increased the rate of TLR compared with clinical follow-up alone.16 Second, with increasing duration of follow-up mortality comprises a relatively higher proportion of overall events, which may reflect the overall baseline risk of the patients analysed. Nevertheless, the 5-year mortality of 14.0% is comparable to that seen in with current everolimus-eluting metallic stents (14.8%) or sirolimus-eluting stents (14.7%) used in a cohort with similar characteristics.15
In terms of late safety results with BVS, the ABSORB II trial included a total of 501 patients randomly (1:1) assigned to treatment with either BVS or an everolimus-eluting DES. Neither the coprimary endpoint (superior vasomotor reactivity nor noninferior late luminal enlargement after 3 years in the BVS group) were met.17 Furthermore, the definite scaffold thrombosis rate after 4 years was 2.6% in the BVS group (vs 0.0% in the DES group), without significant further increase between 3 and 4 years.18
To date, the largest randomized study with available 5-year follow-up is the ABSORB III trial, in which 2008 patients were randomized (2:1) to treatment with either the Absorb BVS or an everolimus-eluting DES. Although the primary endpoint of noninferiority of the BVS in terms of target lesion failure (including cardiac death, target-vessel myocardial infarction and ischemia-driven TLR) after 1 year was met,19 clinical event rates diverged significantly between treatment groups during longer-term follow-up, with higher event rates in the BVS group, especially with respect to scaffold thrombosis (2.5% vs 1.1%; P=.03).20 A recent individual patient-data meta-analysis included the 5-year outcomes from 4 trials and evaluated 2164 patients treated with BVS and 1225 patients treated with DES. At the 5-year follow-up, target lesion failure was more frequent with BVS than with DES (14.9% vs 11.6%; HR, 1.26; 95%CI, 1.03–1.54; P=.03) and the rate of definite scaffold/ stent thrombosis was significantly higher with BVS than with DES (2.3% vs 0.7%; HR, 3.14; 95%CI, 1.48–6.64; P=.003).21 Clinical follow-up data with BVS beyond 3 to 4 years are sparse. To our knowledge, this registry of unrestricted BVS use is the largest to describe 5-year results to date.
The finding of a high rate of device thrombosis in the present registry is in line with the findings of other studies, with a clustering of events within the first 2 years and a relatively stable thrombosis rate between 2 and 5 years. This was also observed in the above- mentioned meta-analysis and might be an expression of complete dissolution within this time frame.21 The overall high incidence of BVS thrombosis may be explained by the interplay of a number of different factors. First, the thicker stent struts and lesser degree of acute gain seen with BVS compared with DES provides a milieu predisposing to a higher risk of device failure.22–24 Moreover, related to these factors, suboptimal device deployment likely also plays a contributory role.25 In the present study, the rate of lesion predilatation was high (97.7%), although postdilatation was performed in a lower proportion of lesions (71.5%). In addition, preclinical studies investigating reendothelialization following BVS implantation in healthy rabbit iliac arteries have shown delayed arterial healing compared with thin-strut DES.22
Second, evidence has emerged that the clinical course in the late scaffold degradation phase is somewhat unpredictable and is not as benign as had been postulated. Case reports have shown that scaffold degradation with prolapse of struts into the vessel lumen may occur late after treatment and that this might be a trigger for late device thrombosis.26 This scaffold discontinuation with malapposition was also observed in our study and in another registry examining optical coherence tomography findings in cases of device failure.27 These prolapsed BVS particles or their degradation products may also be a nidus of BVS thrombosis. Indeed, BVS struts appear to be inherently more thrombogenic relative to contemporary thin-strut DES.22 Moreover, invasive surveillance imaging during follow-up does not seem to identify patients at risk for subsequent thrombotic occlusion.28
Third, the occurrence of late acquired coronary evaginations after BVS implantation has been reported 12 months after implantation in an optical coherence tomography surveillance study of 90 patients.29 The hypothesized causes of this imaging phenomenon were thought to be related to undersizing of the scaffolds at the index procedure and late acquired malapposition secondary to vascular toxicity and inflammation, respectively. These prolapsed BVS particles or their degradation products may also be a nidus of BVS thrombosis. Thus, in view of these factors, in future studies with novel polymeric BVS, prolongation of the dual antiplatelet therapy is likely advisable until further evidence from dedicated long-term follow-up studies are available. This is in keeping with recommendations from a Task Force on the evaluation of BVS30 and with European clinical practice guidelines.31
Finally, in the multivariate analysis, a predictor of the primary endpoint was BVS overlap. Indeed, a recent optical coherence tomography study showed more pronounced neointimal hyperplasia in the region of BVS overlap,32 which potentially contributed to BVS failure. Furthermore, higher age was associated with the incidence of the primary endpoint, which is also a well-known risk factor for adverse events after stenting with DES.33 Comparable to our study, it has also been shown, that BVS perform better in women, possibly related to less complex coronary artery disease.34,35
LimitationsA number of limitations should be taken into account when interpreting the results of this analysis. The study is modest in size and is limited by its nonrandomized, observational nature. The decision to use a BVS was at the discretion of the interventional cardiologist and thus a selection bias cannot be ruled out. The study enrolled consecutive patients including early experience with the BVS technology, before modification in implantation technique became routine (eg, systematic postdilatation), which is reflected by a postdilatation rate of 71.5% in our study. Such recommendations were made during the enrolment period. Although not complete, the systematic angiographic follow-up may have increased the rate of TLR beyond what might have been observed with clinical follow-up alone. Data on long-term antiplatelet therapy and bleeding events were not captured systematically and thus their influence on clinical outcomes remains uncertain. Finally, systematic intravascular imaging was not performed in patients with BVS thrombosis or TLR, which may have helped to identify the cause of BVS failure.
CONCLUSIONSImplantation of everolimus-eluting BVS in routine clinical practice is associated with reasonable antirestenotic performance at short-term follow-up. However, overall clinical event rates during late follow-up to 5 years were high. The rate of scaffold thrombosis was broadly in line with those observed in randomized studies.
CONFLICTS OF INTERESTM. Joner reports receiving consulting fees from Biotronik and Orbus Neich and lecture fees from Boston Scientific; R. A. Byrne reports receiving lecture fees from B. Braun Melsungen AG and Biotronik, and research grants to the institution from Celonova Biosciences. The other authors have no conflicts of interest to declare.
- -
Due to degradation within approximately 3 years, BVS were intended to overcome the long-term limitations of permanent DES. However, randomized trials have shown higher rates of adverse clinical events after implantation of bioresorbable scaffolds than with metallic drug-eluting stents. In particular, a high incidence of thrombotic events later than 1 year after implantation has been observed.
- -
Most randomized studies share strict inclusion and exclusion criteria and thus differ from those treated during daily routine. The present study is the first to report first-time long-term clinical results up to 5 years in patients undergoing unrestricted bioresorbable scaffold implantation. Overall, the rate of adverse clinical events was high. A clustering of scaffold thrombosis was observed within 2 years after BVS implantation.