The long-term prognostic significance of coronary artery dominance pattern in patients with ST-segment elevation myocardial infarction is poorly characterized. We investigated the prognosis of such patients according to whether they had right dominance, left dominance, or codominance.
MethodsThis was a retrospective study of 767 patients, who were admitted to hospital between 2007 and 2012 with ST-segment elevation myocardial infarction and treated with primary percutaneous coronary intervention. We determined the effect of the coronary dominance pattern on all-cause mortality and readmission for infarction, adjusting for mortality as a competing event.
ResultsA total of 80.9% of patients had right coronary dominance, and 8.6% had left coronary dominance. Over 40.8 months’ [interquartile range, 21.9-58.3 months] follow-up, 118 (15.4%) deaths were recorded, of which 39 (5.1%) were in hospital. Mortality for right dominance, left dominance, and codominance was 7.1%, 36.4%, and 13.8% (P ¿ .001), respectively. Cause of death was cardiovascular in 7.1%, 21.2%, and 2.4%. On Cox multivariate analysis, left dominance was significantly associated with mortality (hazard ratio = 1.76; P = .02). Taking “coronary dominance” into account in prediction of risk of death improved the discrimination and calibration capacity of GRACE (Global Registry of Acute Coronary Events) scoring. At follow-up, 9.3% (71 patients) had reinfarction. On multivariate analysis, left dominance was an independent predictor of reinfarction (subhazard ratio = 2.06; P = .01).
ConclusionsIn ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention, left coronary artery dominance confers a higher risk of death and reinfarction than right coronary artery dominance, and should be included in prognostic stratification.
Keywords
Left coronary dominance (CD) and codominance are generally described as a variant of normal anatomy, with a left CD prevalence of around 7% to 10% in the general population.1,2 Patients with left CD have a nondominant right coronary artery that supplies blood only to the right ventricle and right atrium, whilst the left ventricle is completely supplied by the left coronary tree. Therefore, in the case of coronary occlusion, patients with left CD have a larger area of myocardium at risk than patients with right CD, which could negatively affect their prognosis.
There are few clinical studies on the prognostic relevance of CD pattern in patients with acute coronary syndrome (ACS). Furthermore, in the setting of acute myocardial infarction (AMI) with ST-segment elevation (STEMI), the importance of CD pattern in long-term prognosis remains poorly characterized, with conflicting data on its prognostic significance.3,4
The aim of our study was to evaluate the relationship between CD pattern, mortality, and readmission for a new AMI (reAMI), adjusting for mortality as a competing event.
METHODSStudy PopulationThis was a retrospective cohort study based on the CardioCHUS registry; a registry that included all consecutive patients admitted with ACS to the Cardiology Service of the Complejo Hospitalario Universitario de Santiago de Compostela (Santiago de Compostela University Hospital Complex) from December 2003 to September 2012 (N = 5532). Our substudy of the CardioCHUS registry ran from July 2007 to September 2012, a period that we consider to represent current management of acute STEMI. It included consecutive patients who had a primary diagnosis of acute STEMI, had available data on CD pattern, and were treated with primary percutaneous coronary intervention (PCI). The initial population of this substudy was composed of 1084 patients with a primary diagnosis of acute STEMI, of whom 769 had primary PCI as their initial treatment. In 2 patients, CD pattern could not be determined, so those patients were excluded. Thus, the final study population consisted of 767 patients.
Definition of Study VariablesAcute STEMI was defined as presence of symptoms along with ST-segment elevation ≥ 1mm in at least 2 contiguous leads or new or presumed new left bundle branch block, and raised cardiac troponin I (except in cases of early death before laboratory measurement).
Coronary lesions detected on invasive coronary angiography were considered significant if stenosis was ≥ 70% on visual assessment as judged by the responsible cardiologist. This percentage is equivalent to a 50% stenosis on a quantitative analysis method.5 Lesions of the left main coronary artery were considered significant if they were ≥ 50%.
Coronary dominance was defined as the coronary artery giving rise to the posterior descending artery and the posterolateral branches. Coronary dominance was classified as right, left, or codominant. The information on CD was obtained by reviewing coronary angiography reports.
Failed PCI was defined as final TIMI (Thrombolysis In Myocardial Infarction) flow < III or residual stenosis > 30%. Left ventricular ejection fraction was quantified during inpatient stay, using echocardiography according to the Simpson method. The study conformed to the principles of the Declaration of Helsinki.
Aims and Follow-upThe aim of this study was to investigate the prognostic effect of CD type on long-term total mortality and cause of death, as well as (nonfatal) reAMI adjusted for mortality as a competing event, in patients with acute STEMI treated with primary PCI.
After discharge, patients were followed up in a specialized ischemic heart disease outpatient clinic and by their general practitioner. Structured follow-up was carried out using the electronic history (IANUS program, unique to the Spanish autonomous community of Galicia), reviewing all medical visits and hospital records and using telephone contact in some cases.
For classification of cause of death, we used the same classification of cause of death as that previously used by our group.6 Death of cardiovascular origin (cardiac and noncardiac vascular) was defined as that due to sudden death, refractory heart failure, ACS, acute aortic syndrome, pulmonary, systemic, or cerebral thromboembolism, or renal vascular disease (renal failure in the absence of glomerulonephropathy or other parenchymal abnormalities). The remaining causes of death available were considered noncardiovascular.
The causes of death were assigned by 2 cardiologists (M. Castiñeira-Busto and E. Abu-Assi) assigned. If there was a discrepancy between the 2 cardiologists, a third cardiologist (J.M. García-Acuña) was consulted. If information was not available, or there was no consensus on the cause of death, the death was included in the group “cause of death unknown or unclassifiable”.
Statistical AnalysisQuantitative variables are expressed as median [interquartile range], because of the lack of Gaussian distribution, and discrete variables are expressed as frequencies and percentages. The baseline population characteristics were stratified by subgroup of right CD, left CD, or codominance. The Kruskal-Wallis test was used for comparison of continuous variables. The chi-square test or Fisher's exact test was used for discrete variables.
The proportion of deaths in each CD category was estimated with Kaplan-Meier curves, and differences were quantified with the log rank test. The adjusted proportion of reAMI was estimated with a cumulative incidence method, and the differences between the three CD subgroups were quantified using Gray's test.7 The association between CD and mortality was analyzed with a Cox regression model. The intrinsic effect of CD on the reAMI rate was evaluated with the Fine and Gray competing risks model.8 The Cox model included variables with P ≤ .20 in the univariate analysis of total mortality and other variables that were considered clinically relevant (anterior AMI, complete revascularization, and sex). Proportionality of risk assumption was evaluated using Schoenfeld residuals analysis, and the functional form of the quantitative variables (log-linear relationship) was determined using fractional polynomials.9 The following variables were considered in the construction of the multivariate Cox model: year of admission, age ≥ 65 years, sex, diabetes mellitus, vascular disease (peripheral arterial disease or stroke/transient ischemic attack), history of neoplasia, anterior AMI, Killip class ≥ II or left ventricular ejection fraction ≤ 40%, hemoglobin on admission, serum creatinine ≥ 1.3mg/dL on admission, complete coronary revascularization, and CD (with “right CD” as the reference category).
Once the initial Cox model was established, it was then simplified, retaining in the model the covariates that had P ¿ .1, or whose sequential exclusion did not produce changes of > 15% in the coefficient of the variable “left CD”. Thus, the simplified multivariate Cox model included 8 parameters: age ≥ 65 years, diabetes mellitus, Killip class ≥ II or left ventricular ejection fraction ≤ 40%, vascular disease, hemoglobin on admission, serum creatinine ≥ 1.3mg/dL on admission, history of neoplasia, and CD.
The discriminatory capacity of the simplified multivariate model, determined using the C statistic for censored data, was 0.854, which represents 97.6% of the predictive power of the complete model composed of 12 variables: year of admission, age ≥ 65 years, sex, diabetes mellitus, vascular disease, history of neoplastic disease, anterior AMI, Killip class ≥ II or left ventricular ejection fraction ≤40%, hemoglobin on admission, serum creatinine on admission ≥ 1.3mg/dL, complete coronary revascularization, and CD.
The AIC and BIC indices (Akaike information criterion and Bayesian information criterion) were 832 and 883, respectively, for the simplified model (vs 1286 and 1352, respectively, for the complete model), which indicates better adjustment using the parsimonious model.
The final multivariate Fine and Gray competing risks model8 included the variables that showed an association, with P ≤ .20, with the event “reAMI”: age ≥ 65 years, arterial hypertension, diabetes mellitus, previous PCI, and Killip class ≥ II or left ventricular ejection fraction < 35%, as well as the variable “sex”. In 85 patients, values for low-density lipoprotein cholesterol were not available, and therefore this covariate was not introduced in the previous model of competing risks, but we repeated the analysis taking this variable into account, to determine the possible effect on incidence of reAMI.
To study the adjusted effect of CD on mortality after hospital discharge, we repeated the mortality analysis, excluding patients who had not survived the hospital phase (n = 39). We also determined if including CD in risk of death stratification offered increased prognostic value compared with GRACE (Global Registry of Acute Coronary Events) scoring for prediction of risk of death after hospital discharge. The GRACE score is composed of 9 prognostic variables (age, history of heart failure, history of AMI, heart rate and systolic blood pressure on admission, ST-segment, serum creatinine on admission, raised markers of myocardial necrosis, and PCI during the index hospital stay). Although it was designed to predict mortality at 6 months, several studies have corroborated its excellent capacity for prediction of risk of death in the longer-term.10,11
We have calculated and reported the P values that were statistically significant as well as the hazard ratios (HR) and sub-HR with their respective 95% confidence intervals (95%CI). The statistical packages STATA 13 and SPSS 22 were used for statistical analysis.
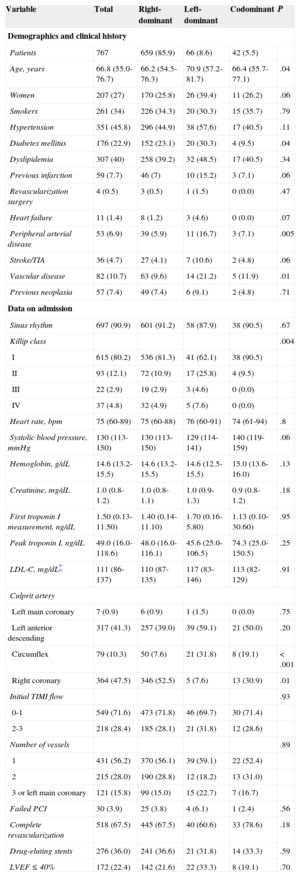
RESULTSSample Characteristics Stratified by Coronary Dominance PatternThe median age was 66.8 years [55.076.7 years] and 27% were women. Patients with left dominance were significantly older than patients with nonleft dominance and generally had a worse cardiovascular risk profile with a higher prevalence of hypertension, diabetes mellitus, history of AMI, vascular disease, and heart failure (Table 1).
Baseline Characteristics
| Variable | Total | Right-dominant | Left-dominant | Codominant | P |
|---|---|---|---|---|---|
| Demographics and clinical history | |||||
| Patients | 767 | 659 (85.9) | 66 (8.6) | 42 (5.5) | |
| Age, years | 66.8 (55.0-76.7) | 66.2 (54.5-76.3) | 70.9 (57.2-81.7) | 66.4 (55.7-77.1) | .04 |
| Women | 207 (27) | 170 (25.8) | 26 (39.4) | 11 (26.2) | .06 |
| Smokers | 261 (34) | 226 (34.3) | 20 (30.3) | 15 (35.7) | .79 |
| Hypertension | 351 (45.8) | 296 (44.9) | 38 (57.6) | 17 (40.5) | .11 |
| Diabetes mellitus | 176 (22.9) | 152 (23.1) | 20 (30.3) | 4 (9.5) | .04 |
| Dyslipidemia | 307 (40) | 258 (39.2) | 32 (48.5) | 17 (40.5) | .34 |
| Previous infarction | 59 (7.7) | 46 (7) | 10 (15.2) | 3 (7.1) | .06 |
| Revascularization surgery | 4 (0.5) | 3 (0.5) | 1 (1.5) | 0 (0.0) | .47 |
| Heart failure | 11 (1.4) | 8 (1.2) | 3 (4.6) | 0 (0.0) | .07 |
| Peripheral arterial disease | 53 (6.9) | 39 (5.9) | 11 (16.7) | 3 (7.1) | .005 |
| Stroke/TIA | 36 (4.7) | 27 (4.1) | 7 (10.6) | 2 (4.8) | .06 |
| Vascular disease | 82 (10.7) | 63 (9.6) | 14 (21.2) | 5 (11.9) | .01 |
| Previous neoplasia | 57 (7.4) | 49 (7.4) | 6 (9.1) | 2 (4.8) | .71 |
| Data on admission | |||||
| Sinus rhythm | 697 (90.9) | 601 (91.2) | 58 (87.9) | 38 (90.5) | .67 |
| Killip class | .004 | ||||
| I | 615 (80.2) | 536 (81.3) | 41 (62.1) | 38 (90.5) | |
| II | 93 (12.1) | 72 (10.9) | 17 (25.8) | 4 (9.5) | |
| III | 22 (2.9) | 19 (2.9) | 3 (4.6) | 0 (0.0) | |
| IV | 37 (4.8) | 32 (4.9) | 5 (7.6) | 0 (0.0) | |
| Heart rate, bpm | 75 (60-89) | 75 (60-88) | 76 (60-91) | 74 (61-94) | .8 |
| Systolic blood pressure, mmHg | 130 (113-150) | 130 (113-150) | 129 (114-141) | 140 (119-159) | .06 |
| Hemoglobin, g/dL | 14.6 (13.2-15.5) | 14.6 (13.2-15.5) | 14.6 (12.5-15.5) | 15.0 (13.6-16.0) | .13 |
| Creatinine, mg/dL | 1.0 (0.8-1.2) | 1.0 (0.8-1.1) | 1.0 (0.9-1.3) | 0.9 (0.8-1.2) | .18 |
| First troponin I measurement, ng/dL | 1.50 (0.13-11.50) | 1.40 (0.14-11.10) | 1.70 (0.16-5.80) | 1.13 (0.10-30.60) | .95 |
| Peak troponin I, ng/dL | 49.0 (16.0-118.6) | 48.0 (16.0-116.1) | 45.6 (25.0-106.5) | 74.3 (25.0-150.5) | .25 |
| LDL-C, mg/dL* | 111 (86-137) | 110 (87-135) | 117 (83-146) | 113 (82-129) | .91 |
| Culprit artery | |||||
| Left main coronary | 7 (0.9) | 6 (0.9) | 1 (1.5) | 0 (0.0) | .75 |
| Left anterior descending | 317 (41.3) | 257 (39.0) | 39 (59.1) | 21 (50.0) | .20 |
| Circumflex | 79 (10.3) | 50 (7.6) | 21 (31.8) | 8 (19.1) | < .001 |
| Right coronary | 364 (47.5) | 346 (52.5) | 5 (7.6) | 13 (30.9) | .01 |
| Initial TIMI flow | .93 | ||||
| 0-1 | 549 (71.6) | 473 (71.8) | 46 (69.7) | 30 (71.4) | |
| 2-3 | 218 (28.4) | 185 (28.1) | 21 (31.8) | 12 (28.6) | |
| Number of vessels | .89 | ||||
| 1 | 431 (56.2) | 370 (56.1) | 39 (59.1) | 22 (52.4) | |
| 2 | 215 (28.0) | 190 (28.8) | 12 (18.2) | 13 (31.0) | |
| 3 or left main coronary | 121 (15.8) | 99 (15.0) | 15 (22.7) | 7 (16.7) | |
| Failed PCI | 30 (3.9) | 25 (3.8) | 4 (6.1) | 1 (2.4) | .56 |
| Complete revascularization | 518 (67.5) | 445 (67.5) | 40 (60.6) | 33 (78.6) | .18 |
| Drug-eluting stents | 276 (36.0) | 241 (36.6) | 21 (31.8) | 14 (33.3) | .59 |
| LVEF ≤ 40% | 172 (22.4) | 142 (21.6) | 22 (33.3) | 8 (19.1) | .70 |
LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; TIMI, Thrombolysis In Myocardial Infarction.
Values are expressed as No. (%) or median (interquartile range).
In addition, left CD patients more often presented to hospital with heart failure. Anterior location of AMI was more frequent in left-dominant patients than in nonleft dominant patients. On angiography, there was a higher proportion of significant 3-vessel/left main coronary artery disease in patients with left dominance, as well as a higher percentage of failed PCI and a lower proportion of complete coronary revascularization compared with patients with right CD or codominance.
On echocardiography, systolic ventricular dysfunction was detected more often in left CD patients.
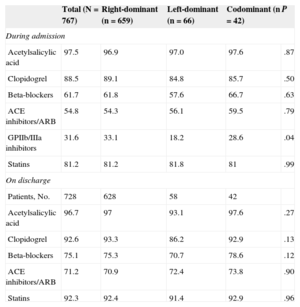
In-patient pharmacological treatment and discharge prescription were not significantly different between the 3 CD subgroups (Table 2).
Pharmacological Treatment in Hospital and on Discharge
| Total (N = 767) | Right-dominant (n = 659) | Left-dominant (n = 66) | Codominant (n = 42) | P | |
|---|---|---|---|---|---|
| During admission | |||||
| Acetylsalicylic acid | 97.5 | 96.9 | 97.0 | 97.6 | .87 |
| Clopidogrel | 88.5 | 89.1 | 84.8 | 85.7 | .50 |
| Beta-blockers | 61.7 | 61.8 | 57.6 | 66.7 | .63 |
| ACE inhibitors/ARB | 54.8 | 54.3 | 56.1 | 59.5 | .79 |
| GPIIb/IIIa inhibitors | 31.6 | 33.1 | 18.2 | 28.6 | .04 |
| Statins | 81.2 | 81.2 | 81.8 | 81 | .99 |
| On discharge | |||||
| Patients, No. | 728 | 628 | 58 | 42 | |
| Acetylsalicylic acid | 96.7 | 97 | 93.1 | 97.6 | .27 |
| Clopidogrel | 92.6 | 93.3 | 86.2 | 92.9 | .13 |
| Beta-blockers | 75.1 | 75.3 | 70.7 | 78.6 | .12 |
| ACE inhibitors/ARB | 71.2 | 70.9 | 72.4 | 73.8 | .90 |
| Statins | 92.3 | 92.4 | 91.4 | 92.9 | .96 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers.
Unless otherwise indicated, data are expressed as percentages.
Over a median follow-up of 40.8 months [21.9-58.3 months], 118 (15.4%) deaths were registered; 39 (5.1%) were in-hospital deaths.
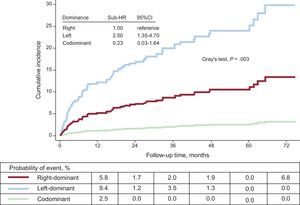
Mortality distribution stratified by CD type showed a monotonic increase from codominance to left dominance (7.1%, 13.8%, and 36.4%; P < .001) (Figure 1A).
Kaplan-Meier curves also showed that the survival curves for the 3 CD types diverged from an early stage, especially for left-dominant patients (Figure 1), and that this divergence continued and even increased over time. When in-hospital deaths (n = 39) were excluded, the differences according to CD type persisted almost unchanged (Figure 1B).
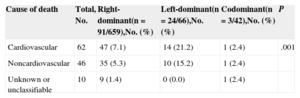
In patients with left dominance, there was a higher proportion of deaths of cardiovascular origin than in those with nonleft dominance (21.2% vs 7.1% for right dominance, and 2.4% for codominance; P = .001) (Table 3).
Cause of Death by Subgroup According to Coronary Dominance Pattern
| Cause of death | Total, No. | Right-dominant(n = 91/659),No. (%) | Left-dominant(n = 24/66),No. (%) | Codominant(n = 3/42),No. (%) | P |
|---|---|---|---|---|---|
| Cardiovascular | 62 | 47 (7.1) | 14 (21.2) | 1 (2.4) | .001 |
| Noncardiovascular | 46 | 35 (5.3) | 10 (15.2) | 1 (2.4) | |
| Unknown or unclassifiable | 10 | 9 (1.4) | 0 (0.0) | 1 (2.4) |
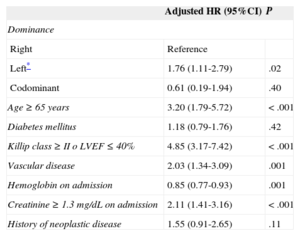
In the multivariate analysis, left CD was positively associated with mortality (HR = 1.76; 95%CI, 1.11-2.79; P = .02) (Table 4); after exclusion of in-hospital deaths, the HR was 1.96 (95%CI, 1.09-3.52; P = .025).
Adjusted Effect of Coronary Dominance Pattern on Total Mortality*
| Adjusted HR (95%CI) | P | |
|---|---|---|
| Dominance | ||
| Right | Reference | |
| Left* | 1.76 (1.11-2.79) | .02 |
| Codominant | 0.61 (0.19-1.94) | .40 |
| Age ≥ 65 years | 3.20 (1.79-5.72) | < .001 |
| Diabetes mellitus | 1.18 (0.79-1.76) | .42 |
| Killip class ≥ II o LVEF ≤ 40% | 4.85 (3.17-7.42) | < .001 |
| Vascular disease | 2.03 (1.34-3.09) | .001 |
| Hemoglobin on admission | 0.85 (0.77-0.93) | .001 |
| Creatinine ≥ 1.3 mg/dL on admission | 2.11 (1.41-3.16) | < .001 |
| History of neoplastic disease | 1.55 (0.91-2.65) | .11 |
95%CI, 95% confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Omnibus test for the variable “coronary dominance” resulted in P = .1 for the complete Cox model composed of 12 variables. After exclusion of the variable “year of admission”, the omnibus test gave P = .08, which was practically unchanged after the exclusion of “sex”. After excluding the variables “anterior acute myocardial infarction” and “complete coronary revascularization”, the omnibus test gave P = .06 and P = .046, respectively.
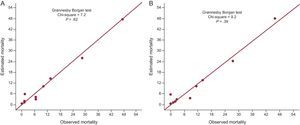
In patients who survived to hospital discharge, GRACE scores for prediction of risk of death after discharge showed an association, as a continuous variable, with mortality (HR = 1.05; 95%CI, 1.04-1.06; P < .001). After adding “CD” to the GRACE score, left CD remained an independent predictor of death (HR = 2.12; 95%CI, 1.15-3.90; P = .015). The C statistic was higher in the model that included CD and GRACE score, compared with GRACE score alone (Hanley-McNeil test, 0.837 vs 0.821; P = .51). Calibration of risk of death after hospital discharge also improved (chi-square value decreased, and the P value of the Grønnesby-Borgan test increased, indicating greater power of calibration, and on visual inspection) when CD was included in the risk estimation (Figure 2).
Patients with intermediate or high risk according to GRACE classification showed a higher risk of death (compared with the low-risk category, HR = 3.72; 95%CI, 1.17-13.92; P = .03; and HR = 23.61; 95%CI, 7.43-75.06; P < .001). Adding “CD” to the GRACE score as a categorical variable improved the C statistic from 0.781 to 0.801 (P = .47).
Relationship Between Coronary Dominance and Acute Myocardial ReinfarctionDuring follow-up, 71 (9.3%) reAMI were recorded. The rates of reAMI according to CD type were 8.6%, 19.7%, and 2.4% for right dominance, left dominance, and codominance, respectively (Figure 3).
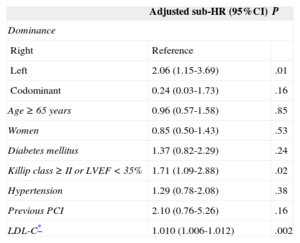
In the multivariate analysis, left CD was an independent predictor of having reAMI at follow-up (sub-HR = 2.06; 95%CI, 1.15-3.69; P = .01) (Table 5).
Adjusted Effect of Coronary Dominance Type on Incidence of Reinfarction Adjusted for Mortality as a Competing Event
| Adjusted sub-HR (95%CI) | P | |
|---|---|---|
| Dominance | ||
| Right | Reference | |
| Left | 2.06 (1.15-3.69) | .01 |
| Codominant | 0.24 (0.03-1.73) | .16 |
| Age ≥ 65 years | 0.96 (0.57-1.58) | .85 |
| Women | 0.85 (0.50-1.43) | .53 |
| Diabetes mellitus | 1.37 (0.82-2.29) | .24 |
| Killip class ≥ II or LVEF < 35% | 1.71 (1.09-2.88) | .02 |
| Hypertension | 1.29 (0.78-2.08) | .38 |
| Previous PCI | 2.10 (0.76-5.26) | .16 |
| LDL-C* | 1.010 (1.006-1.012) | .002 |
95%CI, 95% confidence interval; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Omnibus test resulted in P = .01, and when low-density lipoprotein cholesterol was included in the model, P = .026.
The main finding from this study was that left CD is an independent predictor of death and reAMI in patients with an acute STEMI treated with primary PCI. A pattern of left CD in these patients also confers a greater risk of death of cardiovascular cause. Our data also show that the negative prognostic influence of left CD pattern in the context of acute STEMI starts early and persists, and even increases over time at follow-up. Another finding of our study was the improvement in predictive capacity (improved discrimination and calibration indices) of GRACE scoring in the prediction of mortality after discharge from hospital when CD is taken into account. This is the first study to demonstrate that CD pattern improves predictive capacity of GRACE scoring for risk of death.
There is little information on the prognostic value of CD in patients with ACS in general, and with acute STEMI in particular. In 27 289 patients who underwent cardiac catheterization in the context of ACS, Goldberg et al3 observed that left CD was a predictor of death over a mean follow-up of 3.5 years (HR = 1.18; 95%CI, 1.05-1.34). In their study, the prognostic effect of left CD was more pronounced in patients with STEMI.
In contrast with the findings of Goldberg et al,3 a recent study by Veltman et al4 of 1131 patients with acute STEMI treated with primary PCI revealed that the prognostic importance of left CD was confined exclusively to the first 30 days after the acute event (death, odds ratio = 2.51; 95%CI, 1.11-5.67: combined reAMI or cardiac death, odds ratio = 2.25; 95%CI, 1.09-4.61), and subsequently, (median follow-up 48 months) left CD had a neutral prognostic effect.
In the CathPCI-4 registry, which included 207 926 patients with ACS treated with PCI, left CD, compared with right CD, was an independent predictor of in-hospital mortality (odds ratio = 1.19; 95%CI, 1.06-1.34) in all patients12; however, it was not associated with increased in-hospital mortality in the subgroup of patients with STEMI (odds ratio = 1.2; 95%CI, 0.96-1.30). Unfortunately, the CathPCI-4 registry did not offer information on the long-term prognostic implications of CD pattern.
Thus, our results reinforce the findings of Goldberg et al3 in terms of the prognostic importance of CD pattern in patients with acute STEMI and suggest the need for future studies, given that our results do not support the recent observations by Veltman et al4 regarding the absence of long-term prognostic influence of left CD pattern. We would emphasize that, in comparison with the study by Veltman et al,4 our population had older patients (66 ± 14 vs 61 ± 12 years; P < .001) and a higher burden of comorbidities, which, together with the design differences (The Veltman et al4 study included patients with a first acute STEMI, and excluded patients with previous PCI or coronary revascularization surgery), may have contributed to the differences observed between the studies.
The worse prognosis conferred by left CD in our study may be explained by the fact that right CD has a greater division of coronary vasculature supplying the left ventricle (into 3 “parts”), whereas left CD means that most of the myocardium is essentially dependent on 2 arteries. In fact, in this study, there was increased abnormality of left ventricular function in patients with left CD, which may have led to the worse prognosis observed in those patients compared with those with right CD. However, left CD remained a predictor of worse prognosis after correcting for abnormal left ventricular systolic function.
In this study, there was a higher rate of complete coronary revascularization in patients with right CD (67.5%) than in patients with left CD (60.6%), which was not significant (P = .32) and we think that this finding may be due to the lack of treatment of right coronary artery lesions in left CD patients due to their location in a nondominant vessel.
There may be other mechanisms that explain the worse prognosis associated with left CD in our patients. The classic study by Dodge et al,13 which assessed 83 invasive coronary angiographies showed that the circumflex artery has a larger caliber in patients with left CD. This means that, in patients with left CD, the circumflex artery is more important from the point of view of the coronary circulation, not only because it supplies more coronary branches, but also because of its increased diameter. In fact, Ilia et al14 observed that the rates of cardiogenic shock and in-hospital mortality after acute occlusion of the proximal circumflex artery were higher than those observed after acute occlusion of the left anterior descending artery, which highlights the importance of a dominant circumflex artery that may be responsible for 60% of the blood supply to the left ventricle. Furthermore, Ilia et al14 found that there was a long left anterior descending artery in 87% of patients with left CD compared with 47% of patients with right CD.15 This implies that an affected left anterior descending artery in patients with left CD would produce greater myocardial damage. In our series, 59% of patients with left CD (vs 39% with right CD) had a left anterior descending artery as the artery causing AMI, which, according to the findings of Ilia et al,15 would have caused a greater extent of AMI and ventricular dysfunction, giving a worse prognosis. In our study, we had no data on the length of the left anterior descending artery or on the caliber of the different arteries of the coronary tree, but we did have information on the left ventricular ejection fraction and the severity and extent of the coronary event; nevertheless, left CD maintained its association with mortality and reinfarction. Thus, our study indicates the need for more studies to investigate the underlying causes of the greater fatality of this CD pattern.
Regarding the causal mechanisms of reAMI in our patients with left CD, low-density lipoprotein cholesterol level, which was higher in the left CD subgroup, was an independent predictor of reinfarction, and may explain, at least partly, the increased incidence of reAMI in the presence of left CD. However, other angiographic factors may also have contributed to the association of left CD with reAMI, such as bifurcated and heavily calcified lesions, which occur more often in patients with left CD.16
Given the significant excess of adverse events conferred by left CD in patients with acute STEMI, the indication for intensive pharmacological treatment and a program of specific care,17 as well as carefully planned follow-up, should preferably be considered for patients with acute STEMI and left CD. The search for inducible ischemia related to intermediate lesions in patients with left CD should probably be more active. For example, although stenosis < 50% of the left main coronary artery was not associated with a worse prognosis in an ununselected population of more than 11 000 patients who underwent PCI,18 those types of lesion may be more important in left CD, especially is the trunk is short.19
Our study provides valuable information for long-term risk stratification in patients with acute STEMI. Although the short- and medium-term risks of death and reAMI after ACS are well characterized, the late consequences are still poorly-defined10 and clinicians would appreciate the identification of factors20,21 that predispose to worse long-term prognosis at follow-up, in order to treat or modify them, or at least to counteract their negative prognostic impact. Consideration of CD pattern together with GRACE score provided improved risk-of-death estimations in our series. This improvement was small, and we do not currently know the real impact that it would have on the management of patients with acute STEMI. Nonetheless, we think that CD pattern should be taken into account in the integral process of risk stratification for patients with acute STEMI treated with primary PCI, as in this study it was shown to be an independent predictor of reAMI and death, as well as having contributed to refining the GRACE scoring estimations.
LimitationsFirstly, this was a retrospective study with the limitations inherent to this type of study. We only included patients with acute STEMI who were treated with primary PCI, which may have influenced the prevalence of CD pattern. However, our aim was not to determine the prevalence of CD type, but its prognostic implications. Our findings must be interpreted in the context of acute STEMI treated with primary PCI, and they do not necessarily apply to all patients with acute STEMI. The regression models were at the limit of overadjustment, due to a relatively low number of clinical events in the study.22 In the evaluation of the incremental prognostic utility of CD pattern regarding GRACE scoring, we did not use more sensitive methods such as decision curves,22 which might have provided more information on prognostic utility, or at least another perspective on the net benefit that would have been obtained if CD pattern and GRACE score had been considered together.
Lastly, due to the lack of data on treatment at follow-up, we could not rule out that the differences observed between the 2 groups in our study may have been due, at least in part, to differences in treatment between the 3 groups studied.
CONCLUSIONSIn this contemporary registry of patients with acute STEMI treated with primary PCI, left CD carries a higher risk of mortality and reAMI than right CD. This increased risk begins soon after the acute event and continues, and even increases, throughout follow-up. Mortality of cardiovascular origin was up to 3 times higher in patients with acute STEMI treated with primary PCI with left CD than in those with right CD.
Coronary dominance pattern should be taking into account along with GRACE score for long-term risk-of-death prediction.
CONFLICTS OF INTERESTNone declared.