Conduction disturbances often occur after CoreValve transcatheter aortic valve implantation. The aim was to analyze which cardiac conduction changes occur in patients with aortic stenosis treated with this type of prosthesis.
MethodsA total of 181 patients with severe aortic stenosis treated with this prosthesis and studied by electrocardiography between April 2008 and December 2013 were selected. A subgroup of 137 (75.7%) consecutive patients was studied by intracardiac electrocardiogram before and after prosthesis implantation. The primary endpoint of the study was the need for a permanent pacemaker within 72hours after prosthesis implantation. Numerous variables to predict this possibility were analyzed.
ResultsFollowing implantation, PR and QRS intervals were increased from 173±47 ms to 190±52ms (P < .01) and from 98±22ms to 129±24ms (P < .01), whereas the A-H and H-V intervals were prolonged from 95±39ms to 108±41ms (P < .01) and from 54±10ms to 66±23ms (P < .01). A total of 89 (49%) patients had new-onset left bundle-branch block, and 33 (25%) required a pacemaker within the first 72hours. The independent predictors for a pacemaker were baseline right bundle-branch block and prosthetic depth. Intracardiac intervals had no predictive value. In addition, 13 patients required a pacemaker after 72hours.
ConclusionsCoreValve prosthesis implantation has a high incidence of conduction disturbance, with left bundle-branch block being the most common. A total of 25% of patients required a permanent pacemaker. The need for a pacemaker was related to baseline right bundle-branch block and prosthetic depth.
Keywords
Percutaneous implantation of an aortic valve prosthesis has revolutionized the treatment of aortic stenosis for high-risk surgical patients and has a high success rate and low hospital mortality.1 However, a high percentage of patients require a permanent pacemaker after valve implantation, due to the appearance of advanced atrioventricular block (AVB).2 Because these are older patients with calcified aortic stenosis, many show certain conduction disorders before treatment. In addition, following implantation of a valved stent in the aortic ring and left ventricular outflow tract (LVOT), additional lesions can occur in the atrioventricular node and in the His bundle and its branches that exacerbate these baseline disorders. These outcomes have been investigated by several groups,2 but there is a paucity of information on atrioventricular conduction analysis based on intracardiac electrograms before and after valve implantation.3,4 This study used surface electrocardiograms (ECG) or intracardiac electrograms to analyze the pathophysiology of atrioventricular conduction after CoreValve implantation. Likewise, it analyzed the clinical, anatomic, electrocardiographic, and electrophysiologic factors that predict the need for a permanent pacemaker after percutaneous implantation.
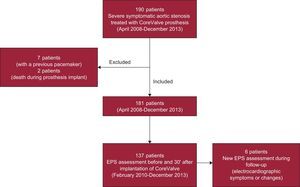
METHODSPatientsA total of 190 patients with severe degenerative aortic valve stenosis were treated between April 2008 and December 2013 by implantation of the Medtronic CoreValve® aortic prosthesis. The study was conducted at a single site, and all patients were assessed by our group. The analysis excluded 9 of 190 patients: 7 because they already had a permanent pacemaker and 2 who died during the procedure (Figure 1).
All patients underwent a protocol-based study that included the following: a) clinical assessment; b) diagnostic catheterization; c) cardiac computed tomography (CT) angiography to analyze the anatomic features of the aortic ring, aortoiliac axis, and LVOT, and d) transesophageal echocardiography to measure aortic ring and LVOT size and to rule out atrial thrombi. Surgical risk was estimated by the logistic EuroSCORE and the Society of Thoracic Surgeons classification. The valve implantation procedure was performed under general anesthesia with echocardiographic imaging using a transesophageal probe. The percutaneous route through the femoral artery was used, except in 2 patients with Leriche syndrome, in whom arterial access was achieved by surgical exposure of the left subclavian artery.
Cardiac Conduction AnalysisAll patients underwent surface ECG at a speed of 25mm/s before and after valve implantation. Rhythm, heart rate, PR and QT intervals, and QRS duration were analyzed; the presence of bundle-branch block, left branch hemiblocks, and advanced AVB was defined according to the diagnostic criteria recommended by the World Health Organization and the International Society and Federation for Cardiology Task Force.5
During the procedure, all patients were monitored by 3-lead ECG tracing, and all received a temporary pacing lead by transjugular access in the right ventricle, for overpacing during aortic valvuloplasty before prosthesis implantation and as prevention in the event of advanced AVB during or after implantation. Surface ECG was performed on arrival at the hospital ward, and continuous ECG monitoring by telemetry was maintained for at least 2 days after the procedure. The pacemaker lead was left in place for the entire prosthesis implantation procedure and the following 72hours, and was withdrawn only if there were no electrical abnormalities requiring permanent cardiac pacing.
Between February 2010 and December 2013, a subgroup of 137 consecutive patients (Figure 1) was studied by EPS before and 30min after valve implantation. A tetrapolar lead was introduced in the His bundle to record the A-H and H-V intervals (measured in milliseconds); A-H intervals < 120ms and H-V intervals < 60ms were considered normal.
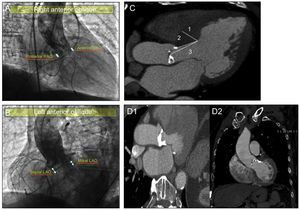
Study of Prosthetic Depth in the Left Ventricle and its Relationship With the Interventricular SeptumParticular emphasis was placed on the valve implant depth analysis and the interventricular septum study. To investigate valve depth, 2 orthogonal angiography views (Figures 2A and B), were taken, namely the left anterior oblique and right anterior oblique.
A: determination of left ventricular outflow tract prosthetic depth measured in right anterior oblique view: “anterior” and “posterior.” B: determination of left ventricular outflow tract prosthetic depth measured in left anterior oblique view: “septal” and “mitral.” C: analysis of interventricular septum by computed tomography angiography; 1, measurement of maximum septal thickness; 2, measurement of distance from end of aortic ring to point of maximum septal thickness; 3, measurement of distance from middle of aortic ring to point of maximum septal thickness. D: analysis of the presence and location of subannular calcification by computed tomography angiography: mitral valve-related subvalvular calcification (D1) and interventricular septum-related subvalvular calcification (D2). LAO, left anterior oblique; RAO, right anterior oblique.
On angiograms, the aortic ring was considered to be the line joining the lowest point of the right coronary cuspid and the lowest point of the left coronary cuspid.6 The different depths were measured as the distance from the aortic ring to the last “rhombus” of the prosthesis introduced in the left ventricle.
The following measurements were taken in the left anterior oblique view: prosthetic depth in relation to the mitral valve and prosthetic depth in relation to the interventricular septum (Figure 2B). The “anterior” and “posterior” prosthetic depths were measured in the right anterior oblique view (Figure 2A). The same digital angiography calibration system (Innova 2100, General Electric; Missouri, United States) was used for all patients.
Computed tomography (Figure 2C) was used to measure the diastolic thickness of the interventricular septum using maximum septal thickness, the distance from the aortic ring to the point of maximum septal thickness, and the distance from the middle of the aortic ring to the point of maximum septal thickness. In addition, CT was used to investigate the presence or absence of calcification at aortic subvalvular level, as well as its location compared with the mitral valve or interventricular septum (Figure 2D).
ObjectivesThe main objective of the study was to analyze the need for a permanent pacemaker within 72hours after CoreValve implantation. The device was considered to be indicated in the case of Mobitz II third- or second-degree AVB with or without symptoms. In particular, the analysis looked at predictors of the need for a pacemaker within the first 72hours after CoreValve aortic prosthesis implantation.
Likewise, the study analyzed atrioventricular conduction changes in patients who underwent this type of percutaneous treatment, using surface ECG (PR and QRS intervals, new cases of right [RBBB] or left [LBBB] bundle-branch block, and complete AVB) and intracardiac electrograms (A-H and H-V intervals).
The secondary objective was to analyze late progression of conduction disturbances that required subsequent implantation of a permanent pacemaker. In this case, the device was considered to be indicated in patients who experienced syncope with serious pulse generation or conduction disorders much later.
Statistical AnalysisAll data are shown as the mean ± standard deviation for quantitative variables or as percentages for categorical variables. The categorical variables were compared using the chi-square test, and the quantitative variables were compared by the Student t test for paired data. Statistical significance was set at P < .05. To predict the need for a pacemaker, a multivariate study was performed by logistic regression using the backward stepwise method, which included all variables that were significant in the univariate analysis. The results are expressed with odds ratios and 95% confidence intervals.
The predictor analysis considered clinical variables (sex, symptoms), surface ECG (baseline presence of atrial fibrillation, RBBB, LBBB, first-degree AVB, bifascicular block or ventricular hypertrophy, and mean PR and mean QRS), structural or anatomic variables (aortic ring by CT and transthoracic echocardiography, presence of aortic subvalvular calcification by CT, site of subvalvular calcification, depth of CoreValve prosthesis implantation in LVOT, and interventricular septum analysis by CT: thickness and morphology) and intracardiac electrograms (A-H and H-V interval).
RESULTSPatients had a mean age of 78 ± 5 years and a mean estimated logistic EuroSCORE of 17.1%±12%. The baseline clinical and diagnostic data for the series are listed in Table 1.
Baseline Patient Characteristics (n=181)
| Age, years | 78±5 |
| Women | 98 (54.1) |
| Cardiovascular risk factors | |
| Diabetes mellitus | 61 (33.7) |
| Hypertension | 120 (67.4) |
| Smoking | 16 (8.9) |
| Dyslipidemia | 91 (50.2) |
| BMI | 28.7±4.5 |
| Dyspnea (NYHA) | |
| NYHA II | 59 (32.5) |
| NYHA III-IV | 118 (65.2) |
| Angina (CCS) | |
| CCS III-IV | 55 (28.7) |
| Syncope | 20 (11.0) |
| Associated heart disease | 58 (32.0) |
| Chronic renal failure (> 2.27 mg/dL) | 22 (12.1) |
| Chronic obstructive pulmonary disease | 22 (12.1) |
| Pulmonary hypertension | 105 (58.0) |
| History of stroke | 6 (3.3) |
| History of cardiac surgery | 12 (6.6) |
| Logistic EuroSCORE, % | 17.1±12 |
| STS score, % | 10.9±10.8 |
| Echocardiographic parameters | |
| Mean TPG, mmHg | 56.3±14.6 |
| Aortic valve area, cm2 | 0.5±0.2 |
| Aortic ring by TEE, mm | 21.3±2.1 |
| Hemodynamic parameters | |
| Peak TPG, mmHg | 72.6±22.6 |
| Aortic ring, mm | 21.5±2.5 |
| EF, % | 56.8±14.7 |
| EF < 40% | 34 (18.7) |
| CT angiography parameters | |
| Aortic ring, mm | 22.6±2.4 |
| Sinus-to-sinus distance, mm | 32.3±3.5 |
| Sinotubular junction, mm | 25.7±3.4 |
| Ascending aorta, mm | 35.5±3.8 |
| Maximum septal thickness, mm | 17.2±3.4 |
| Distance from aortic ring: “maximum septal thickness” | 17.7±5.4 |
| Distance from the center of the valve: “maximum septal thickness” | 25.1±6.1 |
| Presence of subannular calcium | 45 (24.8) |
| Mitral position | 31 (68.9) |
| Septal position | 14 (31.1) |
BMI, body mass index; CCS, Canadian Cardiovascular Society; CT, computed tomography; EF, ejection fraction; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TEE, transesophageal echocardiography; TPG, transaortic pressure gradient.
Slightly over a third of patients (68 [36%]) had some degree of baseline electrocardiographic abnormality, either due to atrioventricular or intraventricular conduction disturbance. In the entire series, 51 (27%) patients had atrial fibrillation, which was paroxysmal in 14.
In the electrocardiographic parameters analyzed, we observed several atrioventricular conduction disorders: 26 patients (14%) had impaired atrioventricular conduction: 6 patients already had a permanent pacemaker due to complete AVB; 21 (11%) had first-degree AVB, 2 of them associated with left anterior hemiblock, 4 with RBBB, and another 4 with LBBB. The mean PR interval of the series was 173±47ms.
A total of 53 (28%) patients had an intraventricular conduction disturbance: at baseline, 17 (9%) had LBBB and 20 (11%) had RBBB, either alone (10 [5.2%]) or associated with other intraventricular conduction disorders (10 patients had bifascicular block). There were also 16 (8.4%) cases of isolated left anterior hemiblock. The mean QRS duration in all patients was 98±22ms.
Electrocardiographic Changes After Valve Implantation. Need for a Permanent Pacemaker Within 72 Hours After ImplantationTable 2 lists electrocardiographic data before and after implantation for all patients: 23 (13%) experienced new-onset first-degree AVB and 89 (49%) had LBBB (in 11 of them, transient and resolved within 72hours after implantation). There was no new-onset RBBB. The mean PR was significantly increased, from 173±47ms to 190±52ms (P < .01), and the mean QRS increased from 98±22ms to 129±24ms (P < .01).
Incidence of Conduction Disorders Before and After CoreValve Prosthesis Implantation
| Electrocardiographic characteristics | Before | After | P |
|---|---|---|---|
| Patients | 181 | 181 | |
| Atrioventricular conduction | |||
| PR interval, ms | 173±47 | 190±52 | < .01 |
| Intraventricular conduction | |||
| QRS complex, ms | 98±22 | 129±24 < 0.01 | |
| Right bundle-branch block | 20 (13 progressed to complete AVB) | 7 (none of new onset) | |
| Left bundle-branch block | 17 (1 progressed to complete AVB) | 105 (89 of new onset, 11 of them transient) | |
| Electrophysiological characteristics | Before | After | |
|---|---|---|---|
| Patients | 137 | 137 | |
| A-H interval, ms | 95±39 | 108±41 | < .01 |
| Mean A-H increase, ms | 13±22 | 13±22 | |
| H-V interval, ms | 54±10 | 66±23 | < .01 |
| Mean H-V increase, ms | 10.5±16 | 10.5±16 |
AVB, atrioventricular block.
The incidence of complete AVB within the first 72hours after valve implantation was 18% (33 patients who required a permanent pacemaker). Among these patients, 15 had no electrocardiographic disturbances before implantation, 3 had isolated left anterior hemiblock, 13 had RBBB (either isolated or with bifascicular block), and 1 had LBBB. One patient with baseline first-degree AVB also experienced complete AVB (Table 3). Seven patients had transient complete AVB that resolved within 72hours postimplantation (4 of them still had definitive LBBB), with no need for a permanent pacemaker (Table 3).
Temporal Progression of the Need for a Permanent Pacemaker
| Early need for pacemaker (< 72hours after CoreValve implantation) | 33 (18.2) |
| Permanent pacemaker | |
| No baseline conduction disturbances | 15 (45.5) |
| Baseline left anterior hemiblock | 3 (9) |
| Baseline right bundle-branch block | 13 (39.5) |
| Baseline first-degree AVB | 1 (3) |
| Baseline left bundle-branch block | 1 (3) |
| Late need for pacemaker (> 72hours after CoreValve implantation) | 13 (7.2) |
| Due to complete atrioventricular block | 9 (69) |
| Before 2 months | 2 |
| After 2 months | 7 |
| Due to syncope with serious conduction disorders | 4 (31) |
| Due to first-degree AVB and left bundle-branch block (months 1 and 10) | 2 |
| Due to left bundle-branch block and normal PR (first month) | 1 |
| Due to first-degree AVB with right bundle-branch block and left anterior hemiblock (first month) | 1 |
AVB, atrioventricular block.
Data are expressed as No. (%).
In the subgroup of 137 patients with intracardiac interval determinations before and after implantation, mean A-H was significantly increased from 95±39ms to 108±41ms (P < .01) and H-V interval similarly increased from 54±10ms to 66±23ms (P < .01) (Table 2).
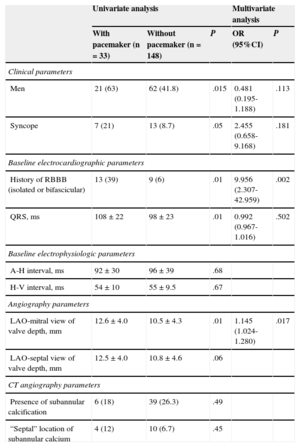
Predictors of Permanent Pacemaker After Valve ImplantationTable 4 lists the predictor variables of pacemaker implantation in the univariate and multivariate analyses. Men were more likely than women to require a pacemaker (P < .02). The need to implant a permanent pacemaker within 72hours was related to baseline QRS width (108±22ms vs 98±23ms; P < .01) and the presence of baseline RBBB, whether isolated or associated with left bundle-branch hemiblock (P < .01), as well as with a history of syncope (P < .05). Patients with baseline LBBB were not more likely to need a pacemaker. Age and the presence of atrial fibrillation were not predictive factors of the need for a permanent pacemaker. Baseline intracardiac intervals also did not predict the need for a pacemaker after implantation.
Predictive Factors of the Need for a Permanent Pacemaker Within 72hours After Valve Implantation
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| With pacemaker (n=33) | Without pacemaker (n=148) | P | OR (95%CI) | P | |
| Clinical parameters | |||||
| Men | 21 (63) | 62 (41.8) | .015 | 0.481 (0.195-1.188) | .113 |
| Syncope | 7 (21) | 13 (8.7) | .05 | 2.455 (0.658-9.168) | .181 |
| Baseline electrocardiographic parameters | |||||
| History of RBBB (isolated or bifascicular) | 13 (39) | 9 (6) | .01 | 9.956 (2.307-42.959) | .002 |
| QRS, ms | 108±22 | 98±23 | .01 | 0.992 (0.967-1.016) | .502 |
| Baseline electrophysiologic parameters | |||||
| A-H interval, ms | 92±30 | 96±39 | .68 | ||
| H-V interval, ms | 54±10 | 55±9.5 | .67 | ||
| Angiography parameters | |||||
| LAO-mitral view of valve depth, mm | 12.6±4.0 | 10.5±4.3 | .01 | 1.145 (1.024-1.280) | .017 |
| LAO-septal view of valve depth, mm | 12.5±4.0 | 10.8±4.6 | .06 | ||
| CT angiography parameters | |||||
| Presence of subannular calcification | 6 (18) | 39 (26.3) | .49 | ||
| “Septal” location of subannular calcium | 4 (12) | 10 (6.7) | .45 | ||
95%CI, 95% confidence interval; CT, computed tomography; LAO, left anterior oblique; OR, odds ratio; RBBB, right bundle-branch block.
In addition, patients were more likely to have AVB if they had prostheses with a greater depth in the LVOT, particularly in the depth analysis based on aortography in the left anterior oblique view in relation to the mitral valve (12.6±4.0mm vs 10.5±4.3mm; P < .01). There was also a stronger tendency to need a pacemaker when the prosthesis had been implanted more deeply in the LVOT in relation to the interventricular septum, although this was not statistically significant (12.5±4.0mm vs 10.8±4.6mm; P < .06). No differences were found in the aortic ring dimensions, the degree of left ventricular hypertrophy, or the presence or location of subvalvular calcification. The CT scans showed that patients with lower interventricular septal thickness in the subaortic portion were more likely to require a pacemaker, but this difference was not statistically significant (17.5±3.1mm vs 16.2±3.3mm; P = .06) (Figure 2C).
In the multivariate analysis, the only independent predictors of the need for a pacemaker were the presence of baseline RBBB (odds ratio=9.95; 95% confidence interval, 2.30-42.95; P = .002) and the valved stent depth in the left ventricle, in its relationship with the mitral valve (odds ratio=1.14; 95% confidence interval, 1.02-1.28; P < .017).
Late Need for a Permanent Pacemaker. Late Analysis of Cardiac Conduction by Electrophysiologic StudyIn 13 patients, there was a late need (beyond the first 72hours after CoreValve prosthesis implantation) of a permanent pacemaker for different reasons: 9 experienced complete AVB at different time points (2 in the first 2 months and 7 afterward) and 4 patients required a pacemaker due to syncope with serious conduction disorders on follow-up (Table 3).
Six patients required an additional repeat EPS after valve implantation, which was performed between day 4 and 20 months after implantation. The new EPS determined the sinus recovery time and the A-H and H-V intervals; the aim was to identify important cardiac conduction changes after valve implantation that required an electrophysiologic explanation and could be used to make a firm decision on permanent pacemaker implantation:
- •
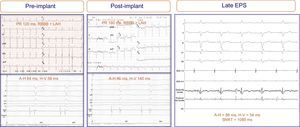
Two patients experienced considerable deterioration in atrioventricular conduction after implantation that returned to normal within 24hours. Once the prosthesis was released, 1 of these patients experienced complete AVB that disappeared within a few hours; repeat EPS at 5 days showed intact atrioventricular conduction and normal A-H and H-V intervals. In the other patient, valve implantation did not affect the baseline ECG (RBBB with left anterior hemiblock), although he did had significant H-V prolongation (58 ms to 140ms); at 7 days, repeat EPS showed complete normalization (H-V, 52ms) (Figure 3). Neither patient received a pacemaker or developed symptoms or arrhythmic events after a follow-up of 29 and 30 months, respectively.
Figure 3.Electrograms and electrocardiograms of a patient with no electrocardiographic abnormalities recorded after prosthesis implantation (bifascicular block was maintained), although significant changes are present in the intracardiac electrogram: H-V interval prolongation (from 58 ms to 140ms). Repeat electrophysiologic study 7 days after implantation showed full normalization of the intracardiac intervals. ADA, anterior descending artery; EPS, electrophysiologic study; LAH, left atrial hemiblock; RBBB, right bundle branch block-bundle of His; RV; right ventricle; SNRT, sinus node recovery time.
(0.48MB). - •
In another 3 patients, prosthesis implantation did not affect the intracardiac intervals, but follow-up showed syncope symptoms or severe asthenia. In 1 of them, repeat EPS showed high-degree AVB with considerable A-H interval prolongation (180ms) at 16 months of follow-up. In the other 2, late EPS showed no changes in atrioventricular conduction, but did reveal sinus node dysfunction at 1 month and 20 months of follow-up, respectively. These 3 patients received a permanent pacemaker.
- •
Following prosthesis implantation, the remaining patient had sinus rhythm with first-degree AVB and significant prolongation of the A-H and H-V intervals. During the first 24hours of progress, complete paroxysmal AVB was observed. Repeat EPS 4 days after implantation showed complete infra-Hisian AVB. A decision was made to implant a permanent pacemaker.
Numerous publications have shown the benefits of percutaneous implantation of an aortic prosthesis for the treatment of older patients with severe aortic stenosis who have symptoms and are at high surgical risk; however, the procedure is not free of mortality and morbidity.1,7 The complications that can appear with this type of prosthesis include AVB with a need for permanent cardiac pacing. In our series, 46 (25%) patients required permanent pacemaker implantation before or after, a figure somewhat lower than the data originating from sites in our setting;3,8 33 (18%) experienced this within 72hours after prosthetic valve implantation. The remaining 13 (7%) were patients who still had atrioventricular and/or intraventricular conduction disorders and experienced complete AVB or symptoms that recommended permanent pacemaker implantation during early follow-up.
Cribier et al9 found that AVB can occur in 3.5% to 4.0% of aortic stenosis cases treated by balloon valvuloplasty after the balloon is inflated in the calcified aortic valve due to balloon-related trauma to the conduction system. With the Edward-SAPIEN prosthesis, the incidence of the need for a permanent pacemaker is around 3.4%,1 but some groups have not had to perform any pacemaker implantations,10 this incidence is much lower than that reported by other groups11,12 after treatment with the CoreValve percutaneous aortic prosthesis (18%-47%). In surgical replacement of the stenotic aortic valve, complete AVB occurs in 3.5% to 8.0% of cases13 and appears to be related to conduction system injury during diseased valve resection and to the surgical sutures.14
However, the conduction disorder that appeared most often was LBBB rather than complete AVB, as described by other authors (40%-71%).8,11 This occurred in 89 patients (49%). This is probably due to anchoring of the valved stent in the interventricular septum, in close relationship with the left bundle branch.15
On occasions, atrioventricular and intraventricular conduction disorders are transient. In our series, this occurred in 7 patients with complete AVB and 11 with new-onset LBBB who regained conduction within 24 hours to 48hours. These cases could be secondary to the edema that occurs in the territory surrounding the conduction system. If this edema is resolved, these disorders should disappear. In 1 of the patients who experienced transient and prolonged (> 24h) complete AVB, repeated EPS performed once the block had disappeared showed normalization of the A-H and H-V intervals and, therefore, pacemaker implantation was not indicated.
This study is the largest consecutive series of patients treated with CoreValve prosthesis in whom the intracardiac electrograms were analyzed before and immediately after prosthesis implantation. The analyses showed slowing of infra-Hisian conduction, consistent with the findings observed by other authors after Edwards-SAPIEN implantation.4 However, the most noteworthy finding of our study was perhaps the increased A-H interval, which was seen in virtually all patients. This finding also indicates some injury to the compact atrioventricular node, but this cannot be explained by the mere anatomic relationship between the aortic valve, LVOT, and conduction system, which is the explanation usually given for cardiac conduction disturbances after valve implantation.16 Because the atrioventricular node is located at some distance from the LVOT, in the right atrial septal aspect, this increase-almost routine-in the A-H interval must have another origin. Perhaps the radial strength exerted by the supporting stent could also compress tissues in areas distant from the aortic valve and the branches of the His-Purkinje system. These data are corroborated by the data reported by another published series,3 which includes a lower number of patients. Likewise, in most patients who experienced complete AVB after implantation, a His electrogram was not achieved in the postimplantation EPS, which indicated considerable injury to the entire conduction system (atrioventricular node and His bundle).
Predictive Factors of the Need for Permanent Pacemaker Within 72 Hours After CoreValve Prosthesis ImplantationClinical PredictorsAn association was found between a history of syncope and the need for a permanent pacemaker after aortic valve implantation. Therefore, the syncope experienced by these patients may not be due only to flow reduction through the stenotic aortic valve, but also to the effects of a diseased conduction system, with previous episodes of paroxysmal AVB and intraventricular block. In our series, just over a third of patients had some baseline atrioventricular and/or interventricular conduction disturbance. In addition, 2 patients who underwent late EPS during follow-up showed some degree of sinus node dysfunction (sinus pause > 8 s and slow atrial fibrillation, with normal H-V intervals). All this indicates that we often treat patients who already have different degrees of conduction disorders that may be worsened after implantation.
Surface and Intracardiac Electrocardiographic PredictorsThe predictive factors for pacemaker implantation include a baseline presence of slower His-Purkinje conduction, seen as wider baseline QRS in the group that required pacemaker implantation, although there were no significant differences in H-V interval among patients who required a permanent pacemaker. This finding could be due to the present of RBBB, in agreement with other studies.16 This is unsurprising, considering that these patients already have a diseased branch of the His system and that the conduction disorder that most frequently occurs in these patients is LBBB, which may cause block of both branches, with the resulting complete infra-Hisian block. In 2003, Koplan et al13 described a risk scale to predict the need for a pacemaker in patients who were scheduled to undergo cardiac surgery. The main predictors of this possibility also include the presence of baseline RBBB, as well as valve surgery, including tricuspid valve surgery, which is closely related to the atrioventricular node and the His-bundle conduction system. The A-H interval assessment was also not a predictor of the need for a permanent pacemaker.
Structural or Anatomic PredictorsPatients with a less thick interventricular septum or with the area of maximum thickness closer to the native aortic valve showed a stronger tendency to experience complete AVB after implantation, but this difference was not statistically significant. An explanation for these findings is that a thicker septum can mean a protective pad for the conduction system. When the maximum septal thickness is closer to the valve plane, it forces contact with the valved stent in the outflow tract, which would explain the possible damage to the His system after implantation (Figure 2C).
Subvalvular calcification and its location had no effect on the need for a permanent pacemaker. These data contradict the findings published by Latsios et al,12 who performed a quantitative analysis of calcification load in the percutaneous valvular device landing zone by CT angiography, rather than a qualitative analysis of subvalvular calcification such as that in our study.
Numerous studies have shown that the prosthetic depth in the left ventricle is a predictive variable of the need for pacemaker implantation.11,12,16 This need is greater when the implant is deeper. Our study found this association when analyzing the prosthetic depth with respect to the mitral valve. Patients were more likely to need a permanent pacemaker when the prosthesis was implanted lower in the LVOT in relation to the interventricular septum, where the bundle of His crosses and divides into its right and left branches. The origin of the left bundle branch is below the commissure located between the right and noncoronary leaflets of the aortic valve, in close relationship to the anterior mitral valve leaflet.15
Among all the variables studied, the only independent factors of the need for a pacemaker in the regression model was the presence of RBBB prior to implantation and the depth achieved by the prosthesis in the left ventricle in relation to the mitral valve. These factors (together with the differing degrees of baseline conduction disorder in most patients) predispose them to worsening after valve implantation.
CONCLUSIONSPatients who undergo percutaneous aortic valve implantation have a high prevalence of atrioventricular and intraventricular conduction disturbances, which tend to worsen after prosthesis implantation. The main conduction disorder that occurs is complete LBBB. Complete AVB with the need for permanent pacemaker occurred in a fourth of patients, mostly within 72hours after implantation.
In addition to causing an intraventricular conduction disturbance due to injury to the His bundle and its branches (shown by systematic H-V interval prolongation), implantation can affect atrioventricular conduction by the compact atrioventricular node (systematic A-H interval prolongation), even though it is at a distant position from the prosthetic structure.
The only independent predictive factors for the need of a permanent pacemaker are baseline RBBB and implantation of an excessively deep valved stent in the LVOT.
CONFLICTS OF INTERESTNone declared.