Coronary artery disease (CAD) and aortic stenosis (AS) often coexist, and they share risk factors with a similar pathogenesis. Randomized studies focusing on surgery vs transcatheter aortic valve replacement (TAVR) have shown that the prevalence of CAD and the patients’ surgical risk have gradually dropped in parallel, from ∼80% in the earliest studies1 to 28% in PARTNER3 and 16% in Evolut Low Risk. In 50% of patients with significant CAD, multivessel involvement is present.2 When CAD is present, the risk of surgical aortic valve replacement is higher, and hence concomitant surgical revascularization is indicated in most of these patients.3 However, when considering a percutaneous therapeutic approach, there are clear differences and several controversial points. First, the impact of CAD on patients undergoing TAVR has not been consistently proven, and results are uneven between the various studies. Second, it is unknown if percutaneous revascularization influences the hypothetical poorer prognosis of these patients (weighed against the benefit of valve disease treatment). Third, in this case, patients do not require simultaneous revascularization and, therefore, the right timing for this procedure is another aspect that should be investigated.
A number of studies have investigated the potential for a deleterious short- or long-term influence of CAD after TAVR. Some have found a negative association between CAD and patient prognosis,4 but the vast majority have observed no impact1,5–7 or only observed an impact in specific subgroups with very extensive CAD.2,8 In a recent article published in Revista Española de Cardiología, Aurigemma et al.9 report on the results of a new single-center, retrospective study now added to this large set of studies attempting to clarify this important issue. These authors evaluated the prognostic impact of at-risk myocardium and the degrees of revascularization in CAD patients treated by TAVR, defining CAD as the presence of stenosis ≥ 70% in a major epicardial coronary vessel or a history of percutaneous or surgical coronary revascularization. To remedy any limitations of prior studies that simply performed dichotomous analyses of CAD (present or absent), the authors calculated the British Cardiovascular Intervention Society myocardial jeopardy score (BCIS-JS) to assess at-risk myocardium before the procedure in patients with CAD. Based on the score, the study population was divided into 3 groups of interest: no CAD (n=223), CAD BCIS-JS ≤ 4 (n=94), and CAD BCIS-JS> 4 (n=86). The primary endpoint was the long-term composite of major adverse events (death, nonfatal acute myocardial infarction, nonfatal stroke, or coronary revascularization). The authors concluded that the group with CAD BCIS-JS> 4 had more events during follow-up and that more complete revascularization before TAVR can improve the prognosis.
Several points should be highlighted from this publication. As mentioned, one of the major limitations of some of the earlier studies is the analysis of CAD as a dichotomous variable, without taking into account its extension, severity, or myocardial repercussions. However, Aurigemma et al. used a more precise approximation of CAD, calculating at-risk myocardium using the BCIS-JS score (range, 0-12). The median BCIS-JS was 4.0 [interquartile range, 3.3-4.7], indicating that more than 75% of the study sample had little at-risk myocardium10 and, consequently, that CAD at the time of TAVR was not very extensive. This finding is consistent with the fact that more than half of the patients only had disease in middle segments (1 or 2 vessels) or isolated disease in the right coronary or secondary vessels. Likewise, this small interquartile range indicates that the vast majority of patients fell within a very narrow range and, therefore, the possibilities of detecting differences based on CAD extension are diminished. Despite this numeric depiction of at-risk myocardium, the authors divided patients with CAD into 2 groups (≤ 4 and> 4). By converting at-risk myocardium into a binary variable, the authors simplified the analysis and made it easier to understand the results, but might have missed a chance to identify how the variable behaves as a prognostic factor, its force of association, or the optimal cut off when analyzing the impact of CAD after TAVR.
Second, the P value provided by the authors is borderline (.049), and because the value is reported for all 3 groups together, it is not possible to identify which one differs from the others. In fact, a visual analysis of the survival curve for the primary endpoint shows that the curves are not proportional over time. The 3-year event rate is similar between the 2 groups with CAD, and the group with no CAD and the group with CAD BCIS-JS ≤ 4 later became comparable. If the analysis had been cut off at 3 years of follow-up, the results might have been different. In view of this asymmetric variation of risk over time between the 3 groups, it is likely that the proportionality assumption is not met and, therefore, the multivariate Cox regression should not be applied. Unsurprisingly, total mortality is slightly higher among patients with CAD, as they have more comorbidities and a worse risk profile. To draw more definitive conclusions, it would be useful to determine whether the cause of death is due to CAD itself and its progression or to other causes with long-term impact.
Third, the inclusion period (9 years) and the follow-up period (3 months to 9 years) were both long. Considerable advances have been made in the percutaneous treatment of aortic valve disease in the past 10 years, with major changes in patient screening, procedure planning and execution, and treatment of complications; in parallel, CAD treatment itself has also changed. The various initial TAVR series had older populations and a high prevalence of CAD, but the potential impact of this on prognosis has been possibly diluted by short-term periprocedural complications and by other illnesses more likely to affect long-term survival. New studies with a younger population and longer follow-up would help determine whether CAD actually impacts survival. In terms of CAD treatment, in the earliest stages, the usual position had been to undertake treatment before or during TAVR. Over the years, the approach has become more conservative, even for CAD in major and proximal vessels, due to technical expertise, better outcomes with the procedure, and newer guidelines. However, the study design did not allow controlling for these factors or for the actual revascularization strategy used. In fact, another analysis by the authors unambiguously revealed the impact of revascularization on clinical events. They concluded that patients with a higher revascularization index have a better prognosis than patients with a lower index. These findings are in contrast with the results of the single randomized study published to date,11 in which patients with CAD and revascularization had a similar 1-year event rate (death or rehospitalization) to that of the unrevascularized group (although the noninferiority criteria were not met), as well as a higher bleeding rate than the unrevascularized group. Aurigemma et al. do not specify the method used to guide the revascularization and made no distinction in their analysis between any percutaneous or surgical revascularization listed in patients’ medical records and percutaneous revascularization immediately before TAVR. Unfortunately, the study did not indicate the type of clinical presentation (presence of symptoms, grade of angina, etc., or if it was a finding in the preintervention study) or the complexity of the coronary lesions. These points reveal the difficulty encountered when assessing this complex condition, which complicates its analysis and the performance of standardized studies to draw firm conclusions.
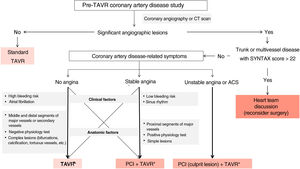
At present, the guidelines recommend considering percutaneous treatment of CAD in patients with an indication for TAVR in arteries with lesions of more than 70% and in proximal segments. However, the therapeutic algorithm can become more complex if clinical and anatomic factors are taken into consideration (figure 1). First, the form of presentation of CAD (acute, chronic, or incidental finding) and the symptoms of aortic valve disease may tip the balance toward one treatment or another. Indeed, a recent study found that only acute coronary syndromes determined a poorer prognosis in these patients,12 whereas revascularization in chronic CAD had no benefit in this clinical setting11 or in others.13 Therefore, the benefit of percutaneous revascularization is still controversial because it is usually not a low-risk procedure in patients with hemodynamically significant AS, which can cause acute decompensation or mid-term risks. Transcatheter revascularization before or during TAVR involves more intensive antiplatelet therapy later, which has been associated with major bleeding events in patients with sinus rhythm and atrial fibrillation after TAVR.11,14,15 The role of coronary physiology is clear in stable CAD, with fewer percutaneous interventions performed routinely in lesions of 30% to 80%. Although certain aspects should be considered when interpreting outcomes in the context of severe AS, especially if it is extremely severe, the hyperemia indexes, particularly those at rest, and the quantitative flow quotient are more precise than visual angiographic analysis to guide revascularization.16 A careful assessment of CAD should consider the therapeutic algorithm because complications are more common in complex lesions, which are also more prevalent in this population. However, revascularization after treating valve disease is also an optional part of a second operation. In this case, it is especially important to consider selecting a replacement valve that facilitates access to the coronaries and an implant that avoids commissure overlap with the coronary ostia. Consequently, the type of percutaneous prosthetic and the implantation technique should also be included in the decision-making algorithm for this complex clinical entity.
Treatment algorithm for coronary artery disease in patients accepted for transcatheter aortic valve implantation. The asterisk indicates selection of a prosthesis with favorable characteristics for a new coronary access (short, intra-annular, large-cell stent) and implantation technique with commissural alignment. ACS, acute coronary syndrome; CT, computed tomography; PCI, percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement.
This Revista Española de Cardiología study is now part of a large set of previous publications analyzing if CAD in patients with AS implies a poorer prognosis, if CAD is simply a marker of risk, or if CAD treatment actually impacts survival. Based on information from clinical trials, it is hard to justify systematic revascularization before TAVR in patients with chronic asymptomatic CAD or with stable symptoms. Until new randomized clinical studies support another strategy, the risk/benefit balance of each intervention plus individualized treatment should guide daily clinical practice.
FUNDINGNo funding.
CONFLICTS OF INTERESTL. Nombela-Franco has been awarded a research grant (INT19/00040) by the Ministry of Science and Innovation of Spain (Carlos III Health Institute) and is a proctor for Edwards Lifesciences and Abbott Vascular. G. Tirado-Conte has been awarded a Rio Hortega research contract (CM21/00091) by the Ministry of Science and Innovation of Spain (Carlos III Health Institute). The other authors have no conflicts of interest related to this article.